Abstract
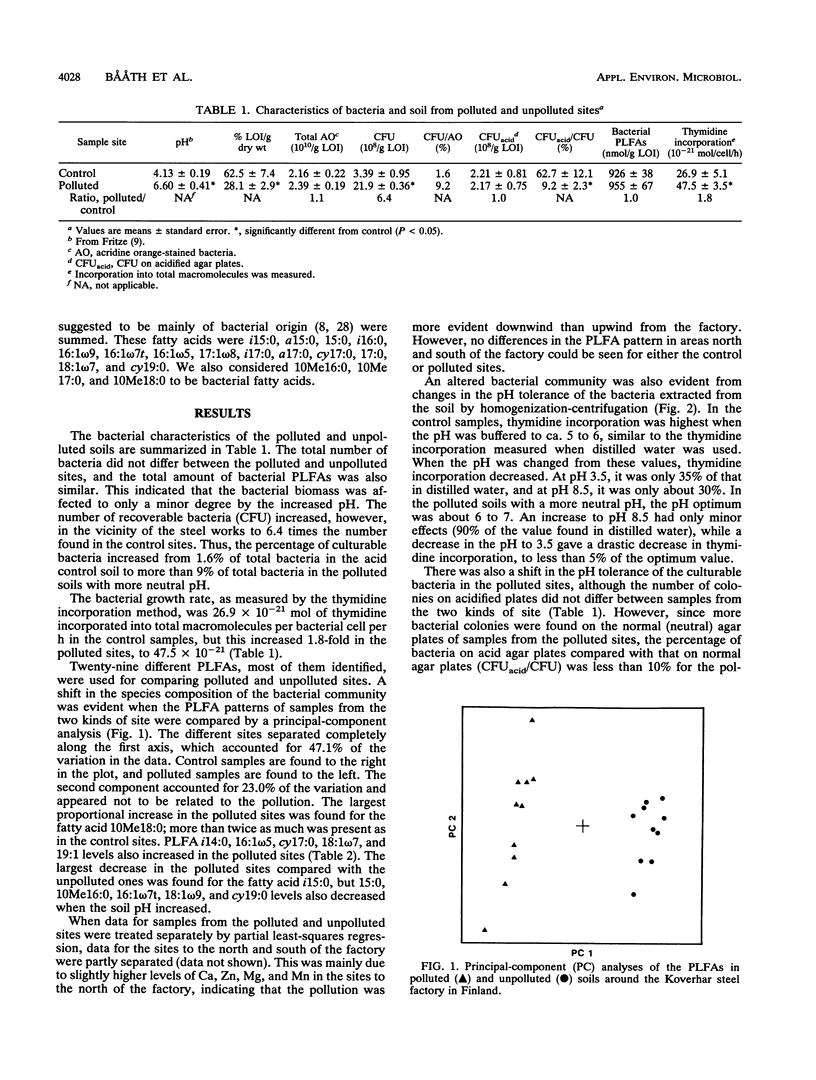
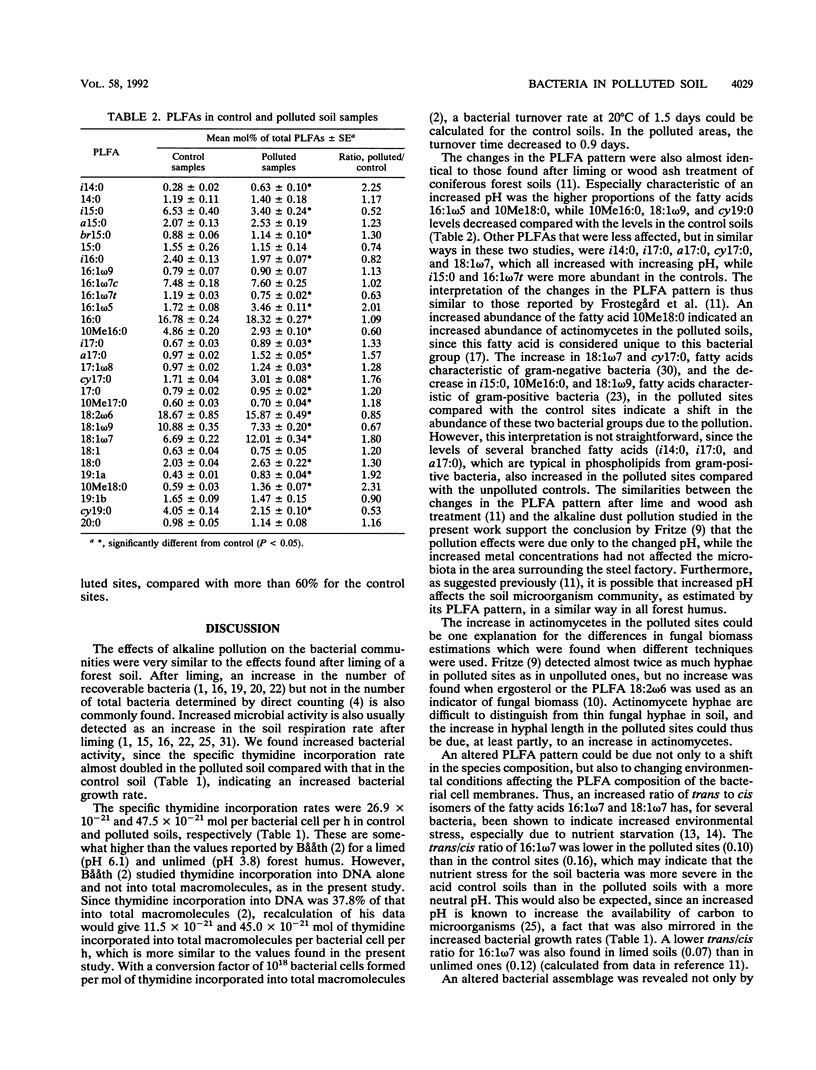
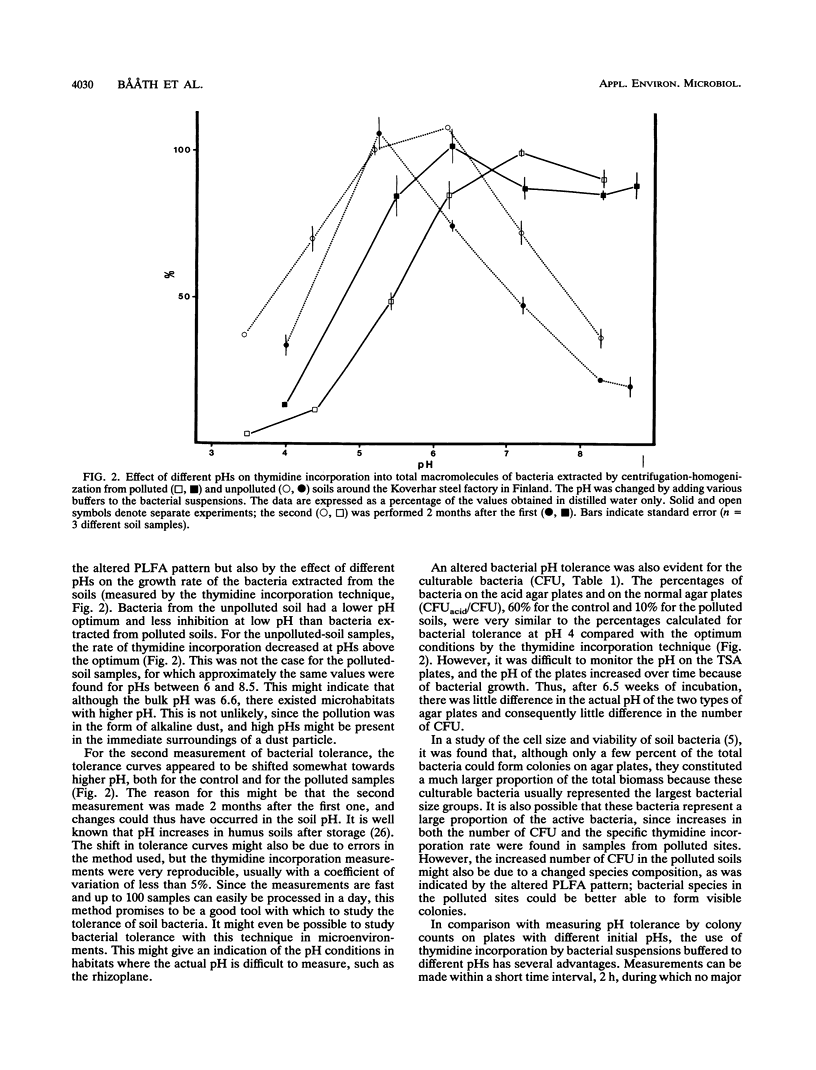
Soil bacterial biomass, phospholipid fatty acid pattern, pH tolerance, and growth rate were studied in a forest area in Finland that is polluted with alkaline dust from an iron and steel works. The pollution raised the pH of the humus layer from 4.1 to 6.6. Total bacterial numbers and the total amounts of bacterial phospholipid fatty acids in the humus layer did not differ between the unpolluted control sites and the polluted ones. The number of CFU increased by a factor of 6.4 in the polluted sites compared with the controls, while the bacterial growth rate, measured by the thymidine incorporation technique, increased about 1.8-fold in the polluted sites. A shift in the pattern of phospholipid fatty acids indicated a shift in the bacterial species composition. The largest proportional increase was found for the fatty acid 10Me18:0, which indicated an increase in the number of actinomycetes in the polluted sites. The levels of the fatty acids i14:0, 16:1ω5, cy17:0, 18:1ω7, and 19:1 also increased in the polluted sites while those of fatty acids 15:0, i15:0, 10Me16:0, 16:1ω7t, 18:1ω9, and cy19:0 decreased compared with the unpolluted sites. An altered pH tolerance of the bacterial assemblage was detected either as a decrease in acid-tolerant CFU in the polluted sites or as altered bacterial growth rates at different pHs. The latter was estimated by measuring the thymidine incorporation rate of bacteria extracted from soil by homogenization-centrifugation at different pHs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Guckert J. B., Hood M. A., White D. C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986 Oct;52(4):794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Ringelberg D. B., White D. C., Hanson R. S., Bratina B. J. Membrane fatty acids as phenotypic markers in the polyphasic taxonomy of methylotrophs within the Proteobacteria. J Gen Microbiol. 1991 Nov;137(11):2631–2641. doi: 10.1099/00221287-137-11-2631. [DOI] [PubMed] [Google Scholar]
- Mai H., Fiedler H. J. Bodenmikrobiologische Untersuchungen an einem Fichtendüngungsversuch im Rauchschadgebiet des Erzgebirges. Zentralbl Bakteriol Naturwiss. 1979;134(8):651–659. [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunlid A., Hoitink H. A., Low C., White D. C. Characterization of bacteria that suppress rhizoctonia damping-off in bark compost media by analysis of Fatty Acid biomarkers. Appl Environ Microbiol. 1989 Jun;55(6):1368–1374. doi: 10.1128/aem.55.6.1368-1374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


