Abstract
Background
Planning public health responses against pandemic influenza relies on predictive models by which the impact of different intervention strategies can be evaluated. Research has to date rather focused on producing predictions for certain localities or under specific conditions, than on designing a publicly available planning tool which can be applied by public health administrations. Here, we provide such a tool which is reproducible by an explicitly formulated structure and designed to operate with an optimal combination of the competing requirements of precision, realism and generality.
Results
InfluSim is a deterministic compartment model based on a system of over 1,000 differential equations which extend the classic SEIR model by clinical and demographic parameters relevant for pandemic preparedness planning. It allows for producing time courses and cumulative numbers of influenza cases, outpatient visits, applied antiviral treatment doses, hospitalizations, deaths and work days lost due to sickness, all of which may be associated with economic aspects. The software is programmed in Java, operates platform independent and can be executed on regular desktop computers.
Conclusion
InfluSim is an online available software http://www.influsim.info which efficiently assists public health planners in designing optimal interventions against pandemic influenza. It can reproduce the infection dynamics of pandemic influenza like complex computer simulations while offering at the same time reproducibility, higher computational performance and better operability.
Background
Preparedness against pandemic influenza has become a high priority public health issue and many countries that have pandemic preparedness plans [1]. For the design of such plans, mathematical models and computer simulations play an essential role because they allow to predict and compare the effects of different intervention strategies [2]. The outstanding significance of the tools for purposes of intervention optimization is limited by the fact that they cannot maximize realism, generality and precision at the same time [3]. Public health planners, on the other hand, wish to have an optimal combination of these properties, because they need to formulate intervention strategies which can be generalized into recommendations, but are sufficiently realistic and precise to satisfy public health requirements.
Published influenza models which came into application, are represented by two extremes: generalized but over-simplified models without dynamic structure which are publicly available (e.g. [4]), and complex computer simulations which are specifically adjusted to real conditions and/or are not publicly available (e.g. [5,6]). The complexity of the latter simulations, however, is not necessary for a reliable description of infection dynamics in large populations [7]. A minimum requirement for a pandemic influenza planning tool is a dynamic modelling structure which allows investigation of time-dependent variables like incidence, height of the epidemic peak, antiviral availability etc. The tool should, on the other hand, be adjustable to local conditions to adequately support the pandemic preparedness plans of different countries which involve considerably different assumptions (Table 1).
Table 1.
Pandemic preparedness plans of some countries
| Attack rate | Outpatients per 100.000 population | Hospitalizations per 100.000 population | Deaths per 100.000 population | Reference | |
|---|---|---|---|---|---|
| Germany | 15% | 15,859 | 437 | 117 | [9] |
| USA | |||||
| - moderate | 30%* | 15,000 | 320 | 77 | [31] |
| - severe | 30%* | 15,000 | 3,666 | 705 | [31] |
| - CDC | 35%* | 17,718 | 277 | 78 | [4] |
| GB | 25% | 25,000 | 140 | 90 | [32] |
| France | 25% | 25,000 | 99 | 20 | [33] |
| Netherlands | 30% | 30,000 | 64 | 26 | [34], [35] |
| Japan | 25%* | 13,077 | 41 | 13 | [36] |
| Canada | 35%* | 16,066 | 359 | 137 | [37] |
Assumed scenarios and outcomes of pandemic preparedness plans. * Gross attack rate (i.e. clinically ill and moderately ill cases).
Here we describe a publicly available influenza pandemic preparedness planning tool [8] which is designed to meet the requirements in preparedness planning. It is based on an explicitly formulated dynamic system which allows addressing time-dependent factors. It is sufficiently flexible to evaluate the impact of most candidate interventions and to consider local conditions like demographic and economic factors, contact patterns or constraints within the public health system. In subsequent papers we will also provide examples and applications of this model for various interventions, like antiviral treatment and social distancing measures.
Implementation
The model is based on a system of 1,081 differential equations which extend the classic SEIR model. Demographic parameters reflect the situation in Germany in 2005, but can be adjusted to other countries. Epidemiologic and clinic values were taken from the literature (see Tables 1, 2, 3, 4, 5, 6 and the sources quoted there). Pre-set values can be varied by sliders and input fields to make different assumptions on the transmissibility and clinical severity of a new pandemic strain, to change the costs connected to medical treatment or work loss, or to simply apply the simulation to different demographic settings. Model properties can be summarized as follows. The mathematical formulation of this model is presented in detail in the online supporting material. The corresponding source code, programmed in Java, and further information can be downloaded from [8].
Table 2.
Age distribution and risk categories
| children | working adults | elderly | ||||
|---|---|---|---|---|---|---|
| 0–5 | 6–12 | 13–19 | 20–39 | 40–59 | 60 + | |
| Population size Na | 5,272 | 6,773 | 7,952 | 25,959 | 29,127 | 24,917 |
A population of N = 100,000 inhabitants of Germany is subdivided according to age a and risk category r. We assume that all age groups are fully susceptible at begin of the outbreak. A fraction of Fa = 6% of all children (age < 20 years) are regarded as being under high risk (r = r1) after an influenza infection whereby the remaining 94% are under low risk (r = r2). The high risk fractions of working adults (ages 20–59) and elderly (ages 60+) are Fa = 14% and Fa = 47%, respectively. Source: [9]
Table 3.
WAIFW matrix
| 0–5 | 6–12 | 13–19 | 20–39 | 40–59 | 60 + | |
|---|---|---|---|---|---|---|
| 0–5 | 169.14 | 31.47 | 17.76 | 34.50 | 15.83 | 11.47 |
| 6–12 | 31.47 | 274.51 | 32.31 | 34.86 | 20.61 | 11.50 |
| 13–19 | 17.76 | 32.31 | 224.25 | 50.75 | 37.52 | 14.96 |
| 20–39 | 34.50 | 34.86 | 50.75 | 75.66 | 49.45 | 25.08 |
| 40–59 | 15.83 | 20.61 | 37.52 | 49.45 | 61.26 | 32.99 |
| 60 + | 11.47 | 11.50 | 14.96 | 25.08 | 32.99 | 54.23 |
The who-acquires-infection-from-whom matrix shows the frequency of contacts (per week per person) between different age classes. Source: [38].
Table 4.
Sojourn times
| Period | average duration | stages | coefficient of variation |
|---|---|---|---|
| Latent period | DE = 1.9 days A | n = 7 | 37.8% A |
| Fully contagious period | |||
| asymptomatic and moderately sick adults | 4.1 days A | m = 19 | 22.9% A |
| others | 7.0 days B | m = 19 | 22.9% C |
| Period of convalescence | DR = 5 days D | j = 9 | 33.3% C |
Distribution of sojourn times (the last two stages of the latent period are used as early infectious period with an average duration of DL = 0.5 days). Sources:A [11], B [39, 40], C assumed, D [41]
Table 5.
Clinical course
| under 20 | 20 to 59 | 60 and older | |
|---|---|---|---|
| Hospitalized fraction ha, r of untreated severe cases | |||
| low risk group (r = r1) | 0.187% | 2.339% | 3.560% |
| high risk group (r = r2) | 1.333% | 2.762% | 7.768% |
| Case fatality da of hospitalized cases | 5.541% | 16.531% | 39.505% |
Independent of age a and risk group r, a fraction ca, r (A) = 33% of infections result in asymptomatic cases, a fraction ca, r (M) = 33.5% become moderately sick and the remaining fraction develops severe disease. An age- and risk-dependent fraction ha, r of untreated patients with severe disease needs hospitalization. An age-dependent fraction da of hospitalized cases dies. Sources: fraction of asymptomatic cases: [11]; 50% of symptomatic cases see a doctor: [9]; hospitalizations per severe case: [9]; case fatality of hospitalized, but untreated patients calculated from [4].
Table 6.
Contagiousness
| Basic reproduction number | R0 = 2.5 |
|---|---|
| Relative contagiousness during the early infectious phase | bL = 50% |
| Relative contagiousness of asymptomatic cases | bA = 50% |
| Relative contagiousness of moderately sick cases | bM = 100% |
| Relative contagiousness of very sick cases | bV = 100% |
| Concentration of the cumulative contagiousness during the first half of the symptomatic period | x50 = 90% |
Sources: Contagiousness of asymptomatic cases: [11]; degree of contagiousness during the early infectious period and equality of the contagiousness of moderately and severely sick cases: assumed.
According to the German National Pandemic Preparedness Plan [9], the total population is divided in age classes, each of which is subdivided into individuals of low and high risk (Table 2). Transmission between these age classes is based on a contact matrix (Table 3) which is scaled such that the model with standard parameter values yields a given basic reproduction number R0. Values for the R0 associated with an influenza strain with pandemic potential are suggested to lie between 2 and 3 [10]. This value is higher than the effective reproduction number which has been estimated to be slightly lower than 2 [11,12]. As a standard parameter, we use R0 = 2.5 which means that cases infect on average 2.5 individuals if everybody is susceptible and if no interventions are performed.
Susceptible individuals who become infected, incubate the infection, then become fully contagious and finally develop protective immunity (Table 4). A fraction of cases remains asymptomatic; others become moderately sick or clinically ill (i.e. they need medical help). Depending on the combination of age and risk group, a fraction of the clinically ill cases needs to be hospitalized, and an age-dependent fraction of hospitalized cases may die from the disease (Table 5). This partitioning of the cases into four categories allows combining the realistic description of the transmission dynamics with an easy calculation of the resources consumed during an outbreak. The degree and duration of contagiousness of a patient depend on the course of the disease; the latter furthermore depends on the age of the patient (Table 5). Passing through the incubation and contagious period is modelled in several stages which allows for realistic distributions of the sojourn times (Table 4). The last two stages of the incubation period are used as early infectious period during which the patient can already spread the disease. Infectiousness is highest after onset of symptoms and thereafter declines geometrically (Table 6). Clinically ill patients seek medical help on average one day after onset of symptoms. Very sick patients are advised to withdraw to their home until their disease is over, whereas extremely sick patients need to be hospitalized and may die from the disease (Table 4). After the end of their contagious period, clinically ill patients go through a convalescent period before they can resume their ordinary life and go back to work (Table 4).
Results
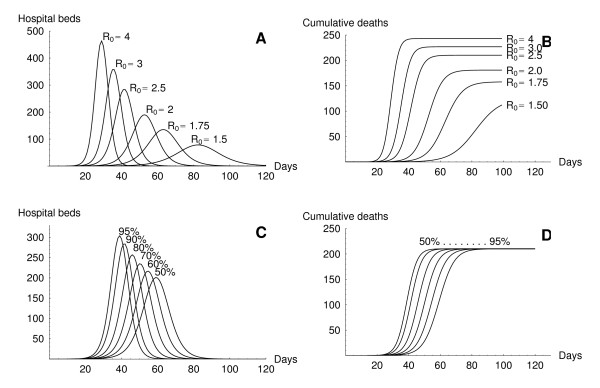
We provide some examples of model output of InfluSim [8], version 2.0, by means of four sensitivity analyses; further investigations will be presented elsewhere. Figure 1 shows the graphical user interface of the software which is divided into input and output windows. The user may set new values in the input fields or move sliders to almost simultaneously obtain new results for the course of an epidemic in a given population. Figures 2A and 2B show pandemic waves which result from varying the basic reproduction number from 1.5 to 4.0. Using the standard parameter values as given in Tables 2, 3, 4, 5, 6 and omitting all interventions in a town of 100,000 inhabitants results in a pandemic wave which lasts for about ten weeks (Figure 2A, with R0 = 2.5). The peak of the pandemic wave is reached after six to seven weeks, with a daily incidence of up to 2,340 influenza patients seeking medical help, with up to 280 hospital beds occupied by influenza cases and with up to 14,000 out of 60,000 working adults unable to go to work because of illness or convalescence. These results depend on the assumptions concerning the yet unknown contagiousness and pathogenicity of the virus. Figures 2C and 2D show how the shape of the curves depends on the course of contagiousness: the pandemic wave proceeds relative slowly if the contagiousness does not change during the infectious period (x50 = 50%), but proceeds quickly if the contagiousness is highest after onset of symptoms and decreases thereafter (x50 > 50%).
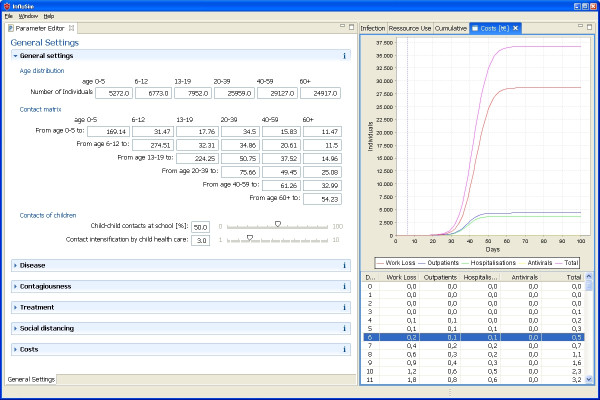
Figure 1.
InfluSim user interface. Graphical user interface of InfluSim. Parameter values can be varied within different tabs (left hand side), divided into General settings (demography by age and risk group, contact matrix, economics), Disease (sojourn times, symptoms, hospitalizations, case fatality), Contagiousness (R0, infectivity over time and by disease severity), Treatment (therapeutic window, treatment schedules, antiviral properties), Social distancing (isolation schedules, general contact reduction, closing day care centres and schools, cancelling mass gatherings) and Costs (work loss, hospitalization, treatment). Time-dependent model output (right hand side) visualizes Infection prevalence (susceptible, exposed, asymptomatic, moderately sick, severely sick, dead, immune), Resource use (work loss, outpatients, hospital beds, antivirals), Cumulative numbers of the latter, and Costs.
Figure 2.
InfluSim output. Examples of InfluSim output for a population of 100,000 citizens. A: Number of hospital beds required during an influenza pandemic for values of R0 ∈ {1.5, 1.75, 2, 2.5, 3, 4}. B: Cumulative number of deaths for values of R0 as in A. C: Number of hospital beds for values of x50 ∈ {50, 60, 70, 80, 90, 95%} (e.g. x50 = 95% means that 95% of the cumulative contagiousness is concentrated during the first half of the contagious period, see Table 6). D: Cumulative number of deaths for values of x50 as in C. All other parameters as listed in Tables 2-6.
Discussion and Conclusion
The influenza pandemic preparedness planning tool InfluSim stands between simple spreadsheet models and sophisticated stochastic computer simulations. It describes a pandemic wave within a homogeneously mixing population like a town or city, but surprisingly produces the same dynamics as individual-based simulations which explicitly consider geographic spread through the US (cf. [6] and [5] with Figure 2 using R0 = 2). Similar observations were made with a simple deterministic compartmental model [7]. Stochastic models are known to behave quasi-deterministically when the simulated population becomes very large.
A further reason for the congruence of complex stochastic and simple deterministic models must lie in the incredibly quick way in which pandemic influenza spreads geographically. Unless being controlled at the place of origin [12,13], a pandemic starting in a far-off country will lead to multiple introductions [14] into the large industrialized nations where it can be expected to quickly spread to neighbouring towns and to rural areas. The large populations which have to be considered susceptible to a pandemic virus and the quick geographic spread tend to diminish the differences between the results of sophisticated individual-based and simple deterministic models.
However, a deterministic model like InfluSim cannot reliably represent effects originating from stochasticity, from effects in small populations, or from heterogeneities. Examples are: (i) a geographically limited spread and fairly effective control measures can imply that the epidemic affects only a small population and thus, may be strongly influenced by stochastic events [15-17]; (ii) transmission which predominantly occurs in households or hospitals, or which is driven by other substantial features of the contact network is not in agreement with the assumption of homogeneous mixing in the deterministic model cannot reliably predict the spread of infection [18-23]. In particular, (iii) super-spreading events can substantially change the course of an epidemic compared to the deterministic prediction [24-27]. Apart from such factors, the predictability of intervention success is generally subject to uncertainties in the choice of parameter values, demanding additional efforts like Bayesian approaches [28] to evaluate the reliability of predictions [29].
Pandemic preparedness plans must consider constraints and capacities of locally operating public health systems. The time-dependent solutions of InfluSim allow assessing peak values of the relevant variables, such as outpatients, hospitalizations and deaths. Various interventions may be combined to find optimal ways to reduce the total number of cases, to lower the peak values or to delay the peak, hoping that at least part of the population may benefit from a newly developed vaccine.
Special care was taken when implementing a variety of pharmaceutical and non-pharmaceutical interventions which will be discussed in subsequent papers. Despite its comprehensible structure, the model does not suffer from over-simplifications common to usual compartment models. Instead of implicitly using exponentially distributed sojourn times, we have implemented realistically distributed delays. For example, the model considers that individuals may transmit infection before onset of symptoms, and that some cases may remain asymptomatic, but still infecting others. Such features have serious implications for the success of targeted control measures.
InfluSim is freely accessible, runs on a regular desktop computer and produces results within a second after changing parameter values. The user-friendly interface and the ease at which results can be generated make this program a useful public health planning tool. Although we have taken care of providing a bug-free program, including the source code, the user is encouraged to treat results with due caution, to test it, and to participate in bug-reports and discussions on the open-source platform [30] which also provides regular updates of InfluSim.
Availability and requirements
Project name: InfluSim version 2.0
Project home page: http://www.influsim.info
Sourceforge: http://sourceforge.net/projects/influsim
Operating systems: Platform independent
Programming language: Java
Other requirements: e.g. Java 1.5 or higher
License: CPL
Any restrictions to use by non-academics: none
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
ME developed the model, MS designed the software, HPD wrote the manuscript and SOB formulated the public health requirements of the software. All authors read and approved the final manuscript.
Appendix: Description of the transmission dynamics of InfluSim version 2.0
Susceptible individuals Sa, r are infected at a rate λa(t) which depends on their age a and on time t. Infected individuals, Ea, r, incubate the infection for a mean duration DE. To obtain a realistic distribution of this duration, the incubation period is modelled in n stages so that progression from one stage to the next one occurs at rate δ = n/DE. The last l incubation stages are regarded as early infectious period during which patients may already spread the infection (this accounts for an average time of lDE/n for the "early infectious period" which is about half a day for the standard set of parameters). After passing through the last incubation stage, infected individuals become fully contagious and a fraction of them develops clinical symptoms. The course of disease depends on the age a of the infected individual and on the risk category r to which he or she belongs: a fraction ca, r(A) becomes asymptomatic (Aa), a fraction ca, r (M) becomes moderately sick (Ma), a fraction ca, r (V) becomes very sick (Va) and the remaining fraction ca, r (X) becomes extremely sick (Xa) and need hospitalization (i.e., ca, r(A) + ca, r (M) + ca, r (V) + ca, r (X) = 1 for each combination of a and r). The rationale for distinguishing very sick and extremely sick cases is that only extremely sick cases can die from the disease and need to be hospitalized; in all other aspects, both groups of severe cases are assumed to be identical. The duration of the fully contagious stage depends on the course of the disease and on the age of the case. Sojourn times are DA, a and DM, a for asymptomatic and moderately sick cases, respectively, and DV, a for both groups of severe cases. To obtain realistic distributions of these sojourn times, the contagious classes are modelled in m stages each so that progression from one stage to the next occurs at rate γA, a = m/DA, a, γM, a = m/DM, a and γV, a, U = m/DV, a, respectively. Severe cases seek medical help on average DD days after onset. Assuming that the waiting time until visiting a doctor is exponentially distributed, we use a constant rate α = 1/DD for doctoral visits. Very sick patients (Va) who visit a doctor are advised to withdraw to their home (Wa) until the disease is over whereas extremely sick cases (Xa) are immediately hospitalized (Ha). A fraction fV (t) of all severe and a fraction fX (t) of all extremely severe cases who visit the doctor within DT days after onset of symptoms are offered antiviral treatment, given that its supply has not yet been exhausted. As our model does not explicitly consider the age of the disease (which would demand partial differential equations), we use the contagious stages to measure time since onset and allow for treatment up to stage ma, T (see below for details). This imposes some variability to the maximum time until which treatment can be given, which may even improve the realism of the model with respect to real-life scenarios. Antiviral treatment reduces the patients' contagiousness by fI percent and it reduces hospitalization and death by fH percent. Extremely sick patients, whose hospitalization is prevented by treatment, are sent home and join the group of treated very sick patients(Wa, T). The remaining duration of disease and contagiousness of treated cases is reduced by fD percent so that their rate of progressing from one stage to the next has to be changed to γV, a, T = m/((1 - fD)DV, a). Extremely sick and hospitalized cases die at rates τa, depending on their age a. Whereas asymptomatic (Aa) and moderately sick patients (Ma) who have passed their last stage of contagiousness are considered healthy immunes (I), very sick and extremely sick patients (classes Va, Wa, U, Wa, T, Xa, Ha, U and Ha, T) first become convalescent (Ca) for an average duration of DC days before they resume their ordinary life. To obtain a realistic distribution of this sojourn time, convalescence is modelled in j stages so that progression from one stage to the next occurs at rate ρ = j/DC. Fully recovered patients who have passed through their last stage of convalescence join the group of healthy immunes I; working adults will go back to work. Further interventions, describing the reduction of contacts, will be discussed after the presentation of the differential equations.
Differential equation model describing the transmission dynamics
Susceptible individuals
Infected individuals who incubate the infection
Asymptomatic infectious individuals
Moderately sick individuals
Very sick individuals who have not yet visited a doctor
Treated very sick individuals
Untreated very sick individuals
Extremely sick individuals who have not yet visited a doctor
Hospitalized and treated cases
Hospitalized, but untreated cases
Contact rates and basic reproduction number
Contact matrix
For the mixing of the age classes, we employ a who-acquires-infection-from whom matrix which gives the relative frequency of contacts of infective individuals of age ai with other people of age as. In this paper, we assume bi-directional contacts (e.g. children have the same total number of contacts with adults as adults with children). Multiplication of this matrix with an appropriate constant scaling factor κ (see below) results in the matrix of crude contact rates .
Contagiousness of the different types of disease
In the absence of interventions, we have to multiply these contact rates with the contagiousness factors bL, bA, bM and bV to obtain the effective contact rates:
during the early infectious period,
of asymptomatic cases,
of moderately sick cases,
of (untreated) very sick cases.
Day care centres and schools
To assess the effect of day care centre and school closing on the transmission of an infectious disease, we have to first make an assumption on what fraction rsch of the contacts among healthy children who are in the same age class occurs in day care centres and schools. The contact rates between very sick or hospitalized children (who do not attend day care centre or school) and other children need, therefore, be reduced to (contact rate between healthy and very sick children in the same age class, i.e. ai = as).
As very sick children have to be taken care of by adults at home or in hospital, their contact rate to adults increases by a factor FHC (contact rate between very sick children of age ai and adults of age as).
Contacts between very sick children and other children in a higher or lower age class remain unchanged: (contact rate between healthy children of age as and very sick children of a different age ai).
Closing of day care centres and schools
Closing day care centres and schools at time t will not necessarily prevent all the contacts that would have happened with other children. During the closing of schools and day care centres, the contact rates between susceptible children of age as and infected children of age ai who are in their late incubation period (), who are asymptomatic (), or who are moderately sick () are reduced by the factor rsch if the children are in the same age class:
where 1sch (t) is a function which indicates when schools and day care centres are opened or closed:
While day care centres and schools are closed, children (age ai) need adult supervision at home. Their contact with susceptible adults (age as) increases by the "child care factor" FCC:
Child care at home also increases the exposure of healthy children (age as) to contagious adults (age ai):
Cancelling of mass gathering events
Cancelling mass gathering events effects only the contacts of adults who are healthy enough to attend such events. Assuming that such an intervention at time t reduces contacts by a fraction rmass, we get for all contacts between susceptible adults of age as and infectious adults of age ai the following contact rates:
where 1mass (t) is a function which indicates when mass gathering events are possible or when they are closed:
As contacts with adults who are too sick to attend such mass gathering events cannot be prevented by this measure it is
.
General reduction of contacts
During some time in the epidemic, the general population may effectively reduce contacts which can be a result of wearing facial masks, increasing "social distance", adopting improved measures of "respiratory hygiene" or simply of a general change in behaviour. This will be implemented in the program by reducing the contacts of susceptible individuals at that time t by factor rgen (t). The adjusted contact rates are:
for cases in the late incubation period,
for asymptomatic cases,
for moderately sick cases,
for very sick cases,
where 1gen (t) is a function which indicates when the population reduces their contacts:
Partial isolation of cases
If cases are (partly) isolated, their contact rates are reduced by factors , and , respectively, resulting in contact rates
for moderately sick cases,
for very sick cases at home,
for hospitalized very sick cases,
where 1iso (t) is a function which indicates when mass gathering events are possible or when they are closed:
The contact rates of cases in the late incubation period and that of asymptomatic cases remain unchanged:
for infected individuals in the late incubation period,
for asymptomatic cases.
Course of contagiousness
To allow for a contagiousness which changes over the course of disease, we multiply each contact rate with a weighting factor whereby k is the stage of contagiousness. This leads to the following contact rates:
for asymptomatic cases in stage k,
for moderately sick cases in stage k,
for very sick cases in stage k,
for hospitalized cases in stage k.
For x = 1, contagiousness is equally high in all stages; for x = 0, only the first stage is contagious; for 0 <x < 1, the contagiousness decreases in a geometric procession. We make the simplifying assumption that contagiousness does not change during the late incubation period
for cases in stage k = n - l,..,n of the incubation period.
Next generation matrix and basic reproduction number
At time t = 0 and in the absence of interventions, the next generation matrix has the following elements
where is the fraction of untreated extremely severe cases who die from the disease (see below for details). The dominant eigenvalue of this matrix is called the basic reproduction number R0. If κ (which determines the value of the contact rates ) is given, the eigenvectors of this matrix can numerically be calculated. The user-specified value of R0 is now used to determine numerically the scaling factor κ. Let be the eigenvector which has the largest eigenvalue R0.
Force of infection
To calculate the force of infection to which susceptible individuals of age as are exposed at time t, we have to first calculate the product of the number of contagious individuals with the corresponding contact rates and then to sum up these products over all ages ai, all risk categories r, all courses of the disease and all stages. Assuming that the contagiousness of cases who have received antiviral treatment is reduced by the factor (1 - fC), the force of infection is given by
Differential equations for various model output
Cumulative number of deaths
Convalescent (but non-contagious) cases
Immune and fully recovered individuals
Number of people who are unable to work because of influenza
where aW denote all age classes of working adults (to avoid infinite contributions to the work loss, the decision was made that cases who die from influenza do not contribute any further to the total work loss).
Cumulative doses of antiviral treatment
Initial values
Using the user-specified numbers of people Na in the age classes and the fractions Fa of people under high risk within each age class (Table 2), we obtain the initial population sizes according to age and risk class: (0) = Na (1 - Fa) and (0) = NaFa. The total population is, therefore, given by .
At time t = 0, one infection is introduced into an otherwise fully susceptible population. To avoid biasing the simulation one way or the other, the initial infection is distributed over all classes, weighted by the probability that an individual in one class acquires the infection (i.e. by the component of the eigenvector of the next generation matrix):
Ak, a (0) = Mk, a (0) = Vk, a (0) = Wk, a, U (0) = Wk, a, T (0) = Xk, a (0) = Hk, a, U (0) = Hk, a, T (0) = 0
Ck, a (0) = 0, D (0) = I (0) = U (0) = T (0) = 0.
Using these initial values, the set of differential equations is solved numerically with a Runge-Kutta method with step-size control.
Abbreviations
Model variables
Transmission variables
Sa, r number of susceptible individuals
Ek, a, r number of incubating individuals (stage k); the last two stages are contagious
Ak, a number of asymptomatic individuals (stage k)
Mk, a number of moderately sick individuals (stage k)
Vk, a number of very sick individuals who have not yet seen a doctor (stage k)
Wk, a, T number of treated very sick individuals (withdrawn to home; stage k)
Wk, a, U number of untreated very sick individuals (withdrawn to home; stage k)
Xk, a number of extremely sick individuals who have not seen a doctor (stage k)
Hk, a, T number of hospitalized and treated individuals (stage k)
Hk, a, U number of hospitalized but untreated individuals (stage k)
Output variables
Ck, a number of convalescent (non-contagious) cases (stage k)
I number of fully recovered and immune cases
D number of people who die of influenza
U number of people who are unable to work because of influenza
T cumulative number of antiviral treatment doses used
Parameters concerning the demography
Na total population size by age class a, whereby a = a1 denotes children, a = a2 denotes adults of working age and a = a2 denotes elderly, respectively.
Fa fraction of the population in age class a which is under high risk from this, Na, r is calculated such that Na, r = Fara
the contact matrix gives the weekly number of contacts between an individual of age class ai with individuals of age class as. From this, the contact rates , , and are calculated as explained above
Parameters concerning the natural history of the disease
Number of stages
n number of stages used to model the latent period
l number of stages used to model the early infectious period
m number of stages used to model the (symptomatic) infectious period
j number of stages used to model convalescence
Sojourn times
DE average duration of the incubation period;
δ is calculated such that δ = n/DE
the last l stages are used as early infectious period
(average duration: DL = DEl/n)
DD average time after onset when a severe case seeks medical help;
α is calculated such that α = 1/DD
DA, a average infectious duration for asymptomatic cases
γA, a is calculated such that γA, a = m/DA, a
DM, a average infectious duration of moderately sick cases
γM, a is calculated such that γM, a = m/DM, a
DV, a average duration of infectivity of untreated very or extremely sick cases;
γV, a, U is calculated such that γV, a, U = m/DV, a
DC average duration of convalescence;
ρ is calculated such that ρ = j/DC
Course of disease
ca, r (A) fraction of asymptomatic infections (given age a and risk r)
sa, r fraction of severe cases among symptomatic ones
ha, r fraction of severe cases who need hospitalization (unless treated) the fraction of infected cases who
- develops moderate disease is ca, r (M) = (1 - sa, r)(1 - ca, r (M))
- becomes bed-ridden at home is ca, r (V) = sa, r (1 - ha, r)(1 - ca, r (M))
- become extremely severe cases is ca, r (X) = sa, rha, r (1 - ca, r (M))
da fraction of untreated extremely severe cases who die;
from this, τa is chosen such that
Parameters concerning the contagiousness of the infection
bL relative contagiousness of cases in the late incubation period
bA relative contagiousness of asymptomatic cases
bM relative contagiousness of moderately sick cases
bV relative contagiousness of severely sick cases
x50 parameter regulating the course of contagiousness
x50 = 1 only the first stage after onset of disease is contagious
0.5 <x50 < 1 contagiousness decreases after onset of disease
x50 = 0.5 equal contagiousness during the whole course of disease
0 <x50 < 0.5 contagiousness increases after onset of disease
from this, x is calculated such that if m is an even number or if m is an odd number, respectively
R0 basic reproduction number; the contact rates , , and are calculated from R0 and from the contagiousness factors as explained above
λa (t) force of infection for susceptible individuals of age a at time t (see calculation above)
Parameters concerning contact reduction
fraction of contacts of moderately sick patients that are prevented by partial isolation
fraction of contacts of very sick patients that are prevented by partial isolation
fraction of contacts of hospitalized patients that are prevented by partial isolation
rgen general fraction of contacts that are prevented at time t
rmass fraction of contacts among (healthy) adults that are prevented by cancelling events of mass gatherings at time t
rsch fraction of contacts among (healthy) children of the same age class that occurs in day care centres or schools
FHC factor by which the contacts between adults and severely sick children increase because of child health care
FCC factor by which the contacts between adults and children increase when children are taken care off at home because schools are closed
Parameters concerning antiviral treatment
Tmax available number of antiviral treatment doses
DT time after onset until when antiviral treatment can still be given; the latest infectious stage ma, T during which treatment can be given, is chosen such that ma, T/γV, a, U ≤ DT ≤ (ma, T + 1)/γV, a, U
fV fraction of severe cases eligible to receive antiviral treatment; treatment will be given only in the user-specified time window and only as long as supplies last:
fX fraction of extremely severe cases eligible to receive antiviral treatment; treatment will be given only in the user-specified time window and only as long as supplies last:
fD fraction by which the duration of infectiousness is reduced by antivirals; γV, a, T is calculated from this such that γV, a, T = m/((1 - fD)DV, a)
fI fraction by which the infectiousness of treated cases is reduced by antivirals
fH fraction of hospitalizations prevented by antiviral treatment
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Martin Eichner, Email: martin.eichner@uni-tuebingen.de.
Markus Schwehm, Email: markus.schwehm@uni-tuebingen.de.
Hans-Peter Duerr, Email: hans-peter.duerr@uni-tuebingen.de.
Stefan O Brockmann, Email: stefan.brockmann@rps.bwl.de.
Acknowledgements
This work has been supported by EU projects SARScontrol (FP6 STREP; contract no. 003824) (HPD) and INFTRANS (FP6 STREP; contract no. 513715) (MS), the MODELREL project, funded by DG SANCO (no. 2003206-SI 2378802) (MS, ME), and by the German Ministry of Health (MS, ME).
References
- Mounier-Jack S, Coker RJ. How prepared is Europe for pandemic influenza? Analysis of national plans. Lancet. 2006;367:1405–1411. doi: 10.1016/S0140-6736(06)68511-5. [DOI] [PubMed] [Google Scholar]
- Smith DJ. Predictability and preparedness in influenza control. Science. 2006;312:392–394. doi: 10.1126/science.1122665. [DOI] [PubMed] [Google Scholar]
- Levins R. The strategy of model building in population biology. American Scientist. 1966;54:421–431. [Google Scholar]
- Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis. 1999;5:659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann TC, Kadau K, Longini IM Jr., Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. Journal of the Royal Society Interface. 2006;3:453–457. doi: 10.1098/rsif.2006.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M, Schwehm M. InfluSim. http://www.influsim.de http://www.influsim.de
- Anonymous. Influenzapandemieplanung: Nationaler Influenzapandemieplan. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2005;48:356–390. doi: 10.1007/s00103-005-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G, Nishiura H, Bettencourt LM. Comparative estimation of the reproduction number for pandemic influenza from daily case notification data. J R Soc Interface. 2007;4:155–166. doi: 10.1098/rsif.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longini IM Jr., Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Longini IM Jr., Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, Halloran ME. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- Mills CE, Robins JM, Bergstrom CT, Lipsitch M. Pandemic Influenza: Risk of Multiple Introductions and the Need to Prepare for Them. PLoS Biol. 2006;3:e135. doi: 10.1371/journal.pmed.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng B, Wang J, Liu J, Wu J, Zhong E. Understanding the spatial diffusion process of severe acute respiratory syndrome in Beijing. Public Health. 2005;119:1080–1087. doi: 10.1016/j.puhe.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RM, Lloyd AL. Infection dynamics on scale-free networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:66112. doi: 10.1103/PhysRevE.64.066112. [DOI] [PubMed] [Google Scholar]
- Roberts MG, Baker M, Jennings LC, Sertsou G, Wilson N. A model for the spread and control of pandemic influenza in an isolated geographical region. Journal of the Royal Society Interface. 2006. [DOI] [PMC free article] [PubMed]
- Ball F, Neal P. A general model for stochastic SIR epidemics with two levels of mixing. Math Biosci. 2002;180:73–102. doi: 10.1016/S0025-5564(02)00125-6. [DOI] [PubMed] [Google Scholar]
- Becker NG, Dietz K. The effect of household distribution on transmission and control of highly infectious diseases. Math Biosci. 1995;127:207–219. doi: 10.1016/0025-5564(94)00055-5. [DOI] [PubMed] [Google Scholar]
- Duerr HP, Schwehm M, Leary CC, De Vlas SJ, Eichner M. The impact of contact structure on infectious disease control: influenza and antiviral agents. Epidemiol Infect. 2007. pp. 1–9. [DOI] [PMC free article] [PubMed]
- Liu JZ, Wu JS, Yang ZR. The spread of infectious disease on complex networks with household-structure. Physica A Physica A. 2004;341:273–280. [Google Scholar]
- Shirley MDF, Rushton SP. The impacts of network topology on disease spread. Ecological Complexity. 2005;2:287–299. doi: 10.1016/j.ecocom.2005.04.005. [DOI] [Google Scholar]
- Wu JT, Riley S, Fraser C, Leung GM. Reducing the impact of the next influenza pandemic using household-based public health interventions. PLoS Med. 2006;3:e361. doi: 10.1371/journal.pmed.0030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Pitchford JW, Plank MJ. An event-based model of superspreading in epidemics. Proc Biol Sci. 2007;274:741–747. doi: 10.1098/rspb.2006.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani AP, May RM. Epidemiology: dimensions of superspreading. Nature. 2005;438:293–295. doi: 10.1038/438293a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LA, Pourbohloul B, Newman ME, Skowronski DM, Brunham RC. Network theory and SARS: predicting outbreak diversity. J Theor Biol. 2005;232:71–81. doi: 10.1016/j.jtbi.2004.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy D, Green N. Optimal intervention for an epidemic model under parameter uncertainty. Math Biosci. 2006;205:297–314. doi: 10.1016/j.mbs.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Colizza V, Barrat A, Barthelemy M, Vespignani A. The Modeling of Global Epidemics: Stochastic Dynamics and Predictability. Bulletin of Mathematical Biology. 2006;68:1893–1921. doi: 10.1007/s11538-006-9077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwehm M, Eichner M. http://sourceforge.net/projects/influsim
- PandemicPlan_US. U.S. Department of Health & Human Services Pandemic Influenza Plan. http://www.hhs.gov/pandemicflu/plan/ http://www.hhs.gov/pandemicflu/plan/
- PandemicPlan_GB. UK Health Department's UK influenza pandemic contingency plan. http://www.dh.gov.uk/PolicyAndGuidance/EmergencyPlanning/PandemicFlu/fs/en http://www.dh.gov.uk/PolicyAndGuidance/EmergencyPlanning/PandemicFlu/fs/en
- Doyle A, Bonmarin I, Levy-Bruhl D, Strat YL, Desenclos JC. Influenza pandemic preparedness in France: modelling the impact of interventions. J Epidemiol Community Health. 2006;60:399–404. doi: 10.1136/jech.2005.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genugten ML, Heijnen ML. The expected number of hospitalisations and beds needed due to pandemic influenza on a regional level in the Netherlands. Virus Res. 2004;103:17–23. doi: 10.1016/j.virusres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- van Genugten ML, Heijnen ML, Jager JC. Pandemic influenza and healthcare demand in the Netherlands: scenario analysis. Emerg Infect Dis. 2003;9:531–538. doi: 10.3201/eid0905.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Ministry of Health, Labour and Welfare, Japan. Action plan of countermeasures against pandemic influenza (Shin-gata influenza taisaku koudou keikaku). Tokyo, Ministry of Health, Labour and Welfare, Japan, 2005 (in Japanese). 2005.
- PandemicPlan_CN. Public Health Agency of Canada. Canadian Pandemic Influenza Plan. http://www.phac-aspc.gc.ca/cpip-pclcpi/index.html http://www.phac-aspc.gc.ca/cpip-pclcpi/index.html
- Wallinga J, Teunis P, Kretzschmar M. Using social contact data to estimate age-specific transmission parameters for infectious respiratory spread agents. American Journal of Epidemiology. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- Bell DM. Non-pharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis. 2006;12:88–94. doi: 10.3201/eid1201.051371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DM. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis. 2006;12:81–87. doi: 10.3201/eid1201.051370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy M, Miles A. The Economic Impact of Influenza in Switzerland - Interpandemic Situation. http://www.bag.admin.ch/themen/medizin/00682/00686/02314/index.html?lang=de# http://www.bag.admin.ch/themen/medizin/00682/00686/02314/index.html?lang=de#