Abstract
Background
To determine whether estradiol-to-progesterone (E2/P) ratios at the time of embryo transfer (ET) have an effect on implantation and pregnancy in IVF cycles.
Methods
239 women consecutively treated by IVF or ICSI were retrospectively analyzed and early luteal serum E2 and P were measured on the day of ET. Transfer occurred after a variable in vitro culture period ranging from 4–7 days after ovulation induction (OI). Following ET, serum E2/P ratios were calculated for clinical pregnancies, preclinical abortions and non-coneption cycles.
Results
Receiver-operator curve analysis demonstrated that the E2/P ratio could differentiate between clinical pregnancies and non-pregnant cycles (area under the curve on OI +4 days = 0.70; 95% CI = 0.60–0.80; p = 0.003, on OI +5 days = 0.76; 95% CI = 0.64–0.88; p = 0.001, OI +7 days = 0.85; 95% CI = 0.75–0.96; p < 0.0001).
Conclusion
These retrospective data may hold prognostic value regarding endometrial receptivity as reflected by E2/P measurements and may help improve IVF treatment outcome. Further prospective studies should be undertaken to confirm these obersveration.
Background
Progesterone (P) and estradiol (E2) are required for successful conception, both to prepare the endometrium for blastocyst implantation and pregnancy. During IVF-ET, controlled ovarian hyperstimulation results in excessive follicular development and supraphysiologic serum concentrations of E2 and P. Such derangements raised concerns about the impact of such abnormalities on the luteal phase and a possible adverse impact on endometrial tissue [1-3]. E2 initiates hypertrophy and hyperplasia of endometrial epithelia, but its role in the luteal phase remains poorly understood. How E2 influences endometrial synchronization and blastocyst implantation is also not well described [4-6]. In contrast, the role of P in the luteal phase is better examined Csapo et al [7,8] showed that luteectomy leads to miscarriage in almost every case if performed before seven weeks of gestational age. P transforms the E2-prepared endometrium into a secretory tissue and creates a hospitable environment for embryo attachment [9].
Although previous research has established that E2 and P regulate events leading to implantation, relatively little is known about their relative proportion in maternal serum during the early luteal phase. In the present study, we retrospectively compared the E2/P ratio in the luteal phase in women undergoing superovulation for IVF-ET who had a successful implantation with those who failed to conceive after such treatment.
Patients and methods
Records from 239 infertile patients attending the assisted reproductive unit at the Department of Obstetrics and Gynecology, General Hospital St. Poelten, Austria, from January 2003 to May 2004 were reviewed. Only those who completed the IVF/ICSI – ET cycle and had a pregnancy test in our laboratory 18 days after ovulation induction were included. Mean (± SD) patient age in this study population was 32.7 ± 3.97 years (range 18–41). Fourty-nine clinical pregnancies were achieved in 239 cycles (118 conventional IVF and 121 ICSI) after ET and a clinical pregnancy rate of 21.0 % per ET was determined. When stratified by diagnostic category, 106 patients had tubal disease (44.4%), 38 had endometriosis (5.4%), 13 had polycystic ovary syndrome (15.9%), 18 had unexplained infertility (7.5%), and 64 had male factor infertility (26.8%). For some patients, more than one infertility factor was assigned. ICSI was indicated for prior failed IVF fertilization(s) and male factor infertility as defined by severe semen abnormalities where semen analysis showed a sperm count of <20 M/mL. Patients were selected on the basis of a stimulation protocol from a computer-generated random number table.
In this study, two controlled ovarian hyperstimulation protocols were used: 61 women were treated with a conventional long protocol using a combination of intranasal buserelin (Suprecur®, Hoechst, Frankfurt, Germany) at a dose of 0.15 mg, 3 times daily from the midluteal phase of the cylce preceding the treatment cycle followed by rFSH (Puregon®, N.V. Organon, Oss, The Netherlands). Additionally, 178 women were treated with rFSH (Puregon®, N.V. Organon, Oss, The Netherlands) starting on day 2 of the menstrual cycle. From day 6–7 of the index cycle, 0.25 mg of ganirelix (Orgalutran®, N.V. Organon, Oss, The Netherlands) was administered daily as a subcutaneous injection up to and including the last day of rFSH administration. Serum concentration of E2 (pg/mL) and transvaginal ultrasound were used to monitor follicular growth. Ovulation was triggered by i.m. administration of 10,000 IU of hCG (Profasi®, Serono, Switzerland) when the mean follicular cohort diameter reached 19 mm. Ovulation induction (OI) was the beginning of the luteal phase and was designated as OI day 0. Oocyte retrieval was carried out transvaginally under ultrasound guidance 34–36 h after OI. Previous studies have described ICSI and IVF procedures in detail [1,10]. Fertilization rate was defined as the proportion of oocytes resulting in two pronuclei (2pn) formation; only metaphase II oocytes were counted in IVF/ICSI cycles. Transfer was carried out 4 days after OI (OI +4 days), 5 days after OI (OI +5 days) or 7 days after OI (OI +7 days). Normally cleaved embryos were replaced under ultrasound guidance using a K-soft 5001 catheter (Cook, Queensland, Australia). All patients had luteal support with Utrogestan vaginal capsules 2 × 100-mg capsules, twice a day (Viatris Pharma, Vienna, Austria) beginning on the day of embryo transfer. Patients with less than an E2 level <1500 pg/mL on the day of oocyte retrieval recieved only one additional luteal support of 1500 IU hCG (Pregnyl®, N.V. Organon, Oss, The Netherlands). Venous blood samples were collected on the morning of oocyte retrieval and on the day of ET. Serum E2 and P concentrations were measured via electrochemiluminescence immunoassay "ECLIA" (Roche Elecsys, Roche Diagnostics, Mannheim, Germany). For E2, inter- and intra-assay coefficients of variation on high concentration control (high E2: 1018 pg/mL) were 2.8 and 1.9%, respectively. For P (high P: 30.2 ng/mL), the inter- and intra-assay coefficients of variation were 5.5 and 2.7%, respectively. The ratio of E2/P was calculated for conception and non-conception cycles as defined below.
Outcome measures
Single serum β-hCG measurement was performed on specimens obtained by peripheral veinpuncture 18 days after OI. Transvaginal ultrasound examination was performed at 8 weeks' gestation to identify clinical pregnancy, defined as the presence of a cardiac action on ultrasound scan. A conception established only on biochemical serum data was defined as preclinical abortion [11]. Supplementary P was continued until 8 weeks of gestation.
Statistical analysis
Statistic Package for Social Sciences (SPSS v 10.0 for Windows, Chicago, IL) software was used for data analysis. Statistical significance was assessed using the Student t-test and χ2 test as appropriate. One-way analysis of variance (ANOVA) was used to test significant difference between groups. Hormonal data were log-transformed to correct for skewness prior to statistical analysis and values in the three groups were compared using the nonparametric Kruskal-Wallis test. Significance was interpreted as p < 0.05. All data were presented as mean ± SD.
From this, receiver operating characteristic (ROC) curves were developed to depict probability of true-positive results (sensitivity) as a function of false-positive results (1 -specificity). Sensitivity and specificity were calculated for all determined ratios of the decision axis and combined with the area under the curve (AUC). The AUC (sensitivity/1 - specificity) format approach was used to confirm test adequacy (AUC near 1) or inadequacy (AUC near 0.5).
Results
The means (±SD) of various clinical parameters for clinical pregnancies, preclinical abortions and for non-conception cycles are presented in Table 1. Mean basal FSH (measured on cycle day 2–4) was 7.39 ± 2.6 IU/L (range 3.0–14). Mean duration of gonadotrophin (rFSH) administration was 10 ± 1.2 days (range 7–13) for the long protocol and 10 ± 1.4 days (range 7–14) for the GnRH-antagonist protocol. The mean peak E2 was 1174.7 ± 828.0 pg/mL (range 164–7196), and mean number of retrieved oocytes was 8.87 ± 6.09 (range 1–28). Only one case of severe OHSS was encountered. The mean number of pre-embryos replaced was 2.5 ± 0.8 (range 1–4). The clinical pregnancy rate per ET was 18.0% (OI +4 days), 21.5% (OI +5 days) and 43.3% (OI +7 days). Fourty-nine (21.0%) had a viable intra-uterine pregnancy at 8 weeks gestations, 27 (11.2%) had an abnormal pregnancy (preclinical abortion) and 163 (67.8%) failed to conceive. There was no influence of the method of fertilization (IVF or ICSI) on the outcome (clinical pregnancies p = 0.668, preclinical abortions p = 0.564 and non-conception cycles p = 0.583; χ2 test).
Table 1.
Comparison between different parameters for clinical pregnancies, preclinical abortions and non-conception cycles.
| Clinical pregnancies | Preclinical abortions | Not pregnant | P valuea | |
| (n = 49) | (n = 27) | (n = 163) | ||
| Age (y) | 32.2 ± 3.6 | 30.8 ± 3.3 | 33.1 ± 4.0 | <0.05 |
| Basal FSH (IU/L) | 7.0 ± 2.6 | 7.2 ± 3.1 | 7.5 ± 2.5 | NS |
| Peak estradiol (pg/mL) | 1325 ± 816 | 1110 ± 586 | 1143 ± 864 | NS |
| Peak progesterone (ng/mL) | 8.5 ± 5 | 10.1 ± 6.2 | 8.2 ± 6.0 | NS |
| No. oocytes/patient | 10.7 ± 6.4 | 10.7 ± 5.7 | 7.9 ± 5.8 | <0.05 |
| Duration of stimulation | 9.8 ± 1.3 | 9.8 ± 1.1 | 10.3 ± 1.4 | NS |
| No. oocytes/fertilized | 7.3 ± 4.3 | 6.9 ± 4.3 | 4.7 ± 3.6 | <.001 |
| No. embryo transfer | 2.8 ± 0.4 | 2.7 ± 0.4 | 2.4 ± 0.9 | NS |
| rFSH dosage (ampoules) | 26.6 ± 6.8 | 31.4 ± 8.0 | 30.8 ± 10.4 | NS |
Note: Values are means ± SD. NS = not significant.
a For the three groups (Kruskal-Wallis test).
There were significant differences when related to the age of the patient (p = 0.005), the number of oocytes retrieved (p = 0.002), and number of fertilized oocytes (p < 0.0001). There were no significant differences in basal FSH, number of gonadotropin units (rFSH) consumed and peak E2 and P on the day of oocyte retrieval among the three groups. The use of a GnRHa long protocol or a GnRH antagonist protocol did not alter the hormonal profile dynamics, the E2/P ratio or clinical pregnancy rate.
Serum (luteal) hormonal parameters at different days of ET (OI +4 days, OI +5 days and OI +7 days) and derived E2/P ratio for clinical pregnancies, preclinical abortions, and non-pregnant cycles are summarized in Table 2. Women with clinical pregnancies had significant higher mean E2/P ratios on OI +4 days (p = 0.01), OI +5 days (p = 0.005) and OI +7 days (p = 0.0001) compared with those who had either a preclinical abortion or failed to conceive (Table 2). Interestingly, mean serum P was higher in women with preclinical abortions compared to clinical pregnancies or non-pregnant cycles, but it did not reach statistical significance.
Table 2.
Luteal phase characteristics of E2, P and the E2/P ratio of clinical pregnancies, preclinical abortions and non-pregnant cycles.
| Clinical pregnancies | Preclinical abortions | Not pregnant | P valuea | |
| E2 on OI +4 days(pg/mL) | 925.4 ± 634.8 | 792.0 ± 586.4 | 812.1 ± 794.5 | NS |
| P on OI +4 days (ng/mL) | 54.0 ± 24.9 | 68.6 ± 60.5 | 56.7 ± 34.4 | NS |
| E2/P Ratio on OI +4 days | 18.2 ± 11.5 | 10.3 ± 8.5 | 11.8 ± 10.0 | 0.010 |
| E2 on OI +5 days (pg/mL) | 1612.5 ± 1118.9 | 1210.4 ± 576.6 | 1257.4 ± 905.2 | NS |
| P on OI +5 days (ng/mL) | 101.4 ± 52.6 | 131.5 ± 66.6 | 110.6 ± 56.3 | NS |
| E2/P Ratio on OI +5 days | 18.1 ± 11.3 | 9.3 ± 6.3 | 8.7 ± 7.9 | 0.005 |
| E2 on OI +7 days (pg/mL) | 2416 ± 787.9 | 2456.5 ± 854.9 | 2032.0 ± 837.4 | NS |
| P on OI +7 days (ng/mL) | 170.9 ± 57.2 | 187.0 ± 28.2 | 198.5 ± 118.4 | NS |
| E2/P Ratio on OI +7 days | 15.4 ± 6.2 | 6.5 ± 7.6 | 5.8 ± 10.7 | <0.001 |
Note: Values are means ± SD. NS = not significant. OI = ovulation induction.
a = For the three groups (Kruskal-Wallis test).
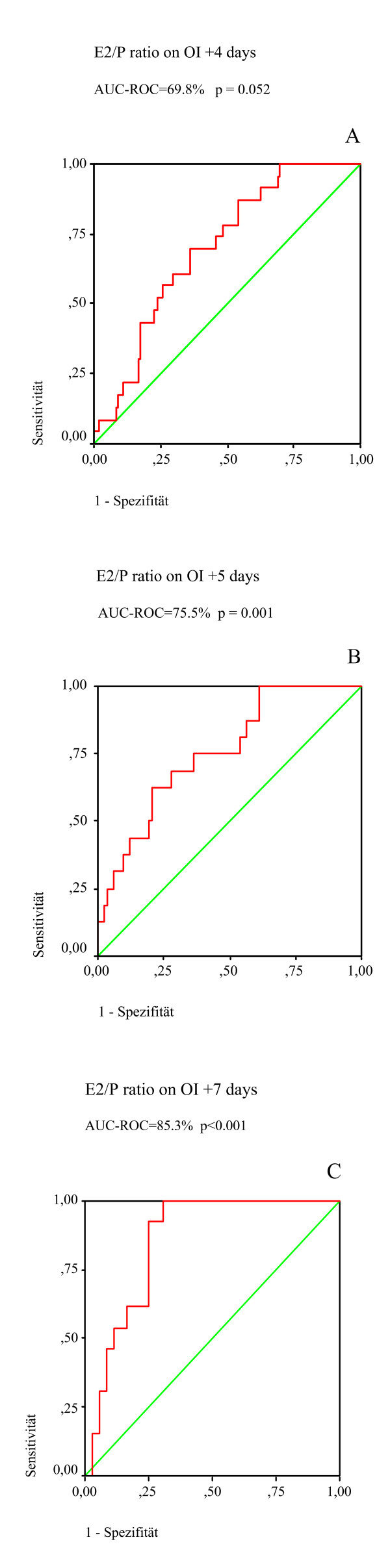
To analyze the prognostic power of E2/P ratio as measured on OI +4 days, OI +5 days and OI +7 days with respect to clinical pregnancy, the AUCROC was determined with ROC analysis (Figure 1). The area under the curve suggests a relationship between E2/P ratio on OI +4 days (0.70; 95% CI = 0.60–0.80; p = 0.003), on OI +5 days (0.76; 95% CI = 0.64–0.88; p = 0.001) and on OI +7 days (0.85; 95% CI = 0.75–0.96; p < 0.0001) and the clinical pregnancy rate.
Figure 1.
ROC-curves for prognostic power of E2/P ratio. Receiver operating characteristic (ROC) curves of the area under the curve (AUC-ROC) for prognostic power of E2/P ratio (A) on OI +4 days, (B) on OI +5 days and (C) on OI +7 days on the clinical pregnancy rate.
Discussion
For normal endometrial morphology to occur, an E2 priming phase is required followed by P. In the pre-GnRH agonist era, the alteration of the E2/P ratio was considered a main cause of luteal-phase inadequacy and IVF failure, possibly mediated by the luteolytic action of E2 [12]. The action of estrogen is required for up-regulation of P receptors. In the follicular and early luteal phases of a normal menstrual cycle, both E2and P receptors are found in glandular and stromal compartements [13]. P antagonizes the proliferative effects of E2 on the endometrial glands by down-regulating estrogen receptors and is followed by a subsequent disappearance of P receptors [14].
Many stimulation cycles in assisted reproduction are associated with failed pregnancy despite the transfer of apparently healthy and morphologically normal embryos. This suggests impairment of endometrial differentiation or receptivity in response to E2 and P may also warrant consideration [15]. In our study, the role of the E2/P ratio at the time of embryo transfer was compared with the pregnancy outcome. Our working hypothesis of this investigation is that a high P level in combination with a low E2 level in the early luteal phase could presage failed implantation. These data suggest that the E2/P ratio on OI +4 days, OI +5 days and OI +7 days are significantly associated with clinical pregnancy rate. Interestingly, a significant higher clinical pregnancy rate could be achieved if blastocysts on OI +7 days were transferred. It seems that, blastocyst transfer allowed the identification of embryos with very high implantation potential [16], and probably a better blastocyst-endometrial epithelium interaction. The behaviour of the blastocyst may be influenced by signals from the endometrium which has been primed with preimplantation ovarian steroids [17].
Specifically, we identified no differences in peak E2 and P on day of oocyte retrieval or in the early luteal (on OI +4 days, OI +5 days and OI +7 days) E2 and P concentrations between pregnant and non-pregnant women. The use of a single luteal E2 and P measurement to predict endometrial receptivity was not useful, although at our center the early detection of a low E2 level did help identify those who were then given supplementary hCG support for corpus luteum rescue. Additionally, a single P value in the early luteal phase was not informative for diagnosing luteal phase defect. Assessment of the E2/P ratio in the early luteal phase provided better prognostic information with relatively higher values of this ratio being associated with a healthy corpus luteum activity and successful implantation.
In this study, patients received both GnRH-agonist and GnRH-antagonist stimulated cycles for controlled ovarian hyperstimulation to prevent premature LH surge. Uniform luteal support consisting of vaginal micronized progesterone starting on the day of ET was given to all patients in order to compensate for possible iatrogenic luteal phase defect [18]. GnRH- agonists are associated with persistent blockage of LH output for at least 10 days following the final dose [2,19]. Prolonged administration of GnRH agonists may also affect ovarian steroidgenesis directly because of the presence of GnRH receptors in the ovary [20]. In contrast, an inhibitory effect of GnRH-antagonist on steroidgenesis may also be postulated [21,22]. Here, we confirmed the present hormonal profile dynamics, and the calculated E2/P ratio was not affected by the treatment protocol used (GnRH agonist vs. antagonist), findings that agree with previous work [23].
Conclusion
We conclude that moderately increased P values in the early luteal phase was associated with higher E2/P ratios and better pregnancy outcomes, whereas a high increase in P values in combination with a decrease in E2values (reflected by a low E2/P ratio) append to indicate poor reproductive outcome. Consequently, this retrospective study implies that in the latter setting the embryo will encounter a poorly receptive endometrium on the day of transfer, resulting in impaired implantation.
To our knowledge, this is the first study specifically evaluating E2/P ratio in relation to IVF outcome. Further study is needed to examine whether the E2/P ratios could be used as a prognostic test to predict which women will have a clinical pregnancy in the setting of advanced reproductive technologies following a COH.
Competing interests
The author(s) declare that they have no competing interests.
Acknowledgments
Acknowledgements
None.
Contributor Information
Irmhild Gruber, Email: ivf@stpoelten.lknoe.at.
Alexander Just, Email: alexander.just@stpoelten.lknoe.at.
Monika Birner, Email: monika.birner@stpoelten.lknoe.at.
Alexander Lösch, Email: alexander.loesch@stpoelten.lknoe.at.
References
- Edwards RG, Steptoe PC, Purdy JM. Establishing full-term pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, Van Waesberghe L, Wisanto A, Van Steirteghem AC. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum Reprod. 1988;3:585–590. doi: 10.1093/oxfordjournals.humrep.a136750. [DOI] [PubMed] [Google Scholar]
- Albano C, Grimbizis G, Smitz J, Riethmuller-Winzen H, Reissmann T, Van Steirteghem A, Devroey P. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotrophin-realising hormone antagonist Cetrorelix. Fertil Steril. 1998;70:357–359. doi: 10.1016/S0015-0282(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Forman R, Fries N, Testart J, Belaisch-Allart J, Hazout A, Frydman R. Evidence for an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertil Steril. 1988;49:118–122. doi: 10.1016/s0015-0282(16)59661-7. [DOI] [PubMed] [Google Scholar]
- Sharara FI, McClamrock HD. Ratio of estradiol concentration on the day of human chorionic gonadotrophin administration to mid-luteal estradiol concentration is predictive of in-vitro fertilization outcome. Hum Reprod. 1999;14:2777–2782. doi: 10.1093/humrep/14.11.2777. [DOI] [PubMed] [Google Scholar]
- Ng Hung Yu E, Shu Biu Yeung W, Yee Lan Lau E, Wai Ki So W, Ho Chung P. A rapid decline in serum oestradiol concentrations around the mid-luteal phase had no adverse effect on outcome in 763 assisted reproduction cycles. Hum Reprod. 2000;15:1903–1908. doi: 10.1093/humrep/15.9.1903. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Weist NG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115:759–765. doi: 10.1016/0002-9378(73)90517-6. [DOI] [PubMed] [Google Scholar]
- Csapo A, Pulkkinen MO, Kaihola HL. The effect of estradiol replacement therapy on early pregnant luteectomized patients. Am J Obstet Gynecol. 1973;115:987–990. doi: 10.1016/0002-9378(73)90073-2. [DOI] [PubMed] [Google Scholar]
- Weitlauf HM. The Physiology of Reproduction. New York: Raven Press; 1994. Biology of implantation; pp. 233–277. [Google Scholar]
- Devroey P, van Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Nygren KG, Adamson D, de Mouzon J, Lancaster P, Mansour R, Sullivan E. The International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary on ART terminology. Fertil Steril. 2006;86:16–19. doi: 10.1016/j.fertnstert.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gore BZ, Cadwell BV, Speroff L. Estrogen-induced human luteolysis. J Clin Endocrinol Metab. 1973;36:615–617. doi: 10.1210/jcem-36-3-615. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:34–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Perrot-Applanat M. Use of immunocytochemistra of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab. 1988;67:80–87. doi: 10.1210/jcem-67-1-80. [DOI] [PubMed] [Google Scholar]
- Paulson RJ, Sauer MV, Lobo RA. Embryo implantation after human in vitro fertilization: importance of endometrial receptivity. Fertil Steril. 1990;53:870–874. doi: 10.1016/s0015-0282(16)53524-9. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens John , Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–3440. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- Lopata A. Molecular aspects of implantation. Blastocyst-endometrial interaction: an appraisal of some old and new ideas. Mol Hum Reprod. 1996;2:519–525. doi: 10.1093/molehr/2.7.519. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Fauser BC. Impact of ovarian hyperstimulation on the luteal phase. J Reprod Fertil. 2000. pp. 101–108. [PubMed]
- Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Folicular and luteal phase characteristics following early cessation of gonadotropin-releasing hormone agonist during ovarian stimulation for in-vitro fertilization. Hum Reprod. 2000;15:43–49. doi: 10.1093/humrep/15.1.43. [DOI] [PubMed] [Google Scholar]
- Clayton RN, Catt KJ. Regulation of pituitary gonadotropin-releasing hormone receptors by gonadal hormones. Endocrinology. 1981;108:887–895. doi: 10.1210/endo-108-3-887. [DOI] [PubMed] [Google Scholar]
- Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BC. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist co treatment. J Clin Endocrinol Metab. 2003;88:4186–4192. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid recepter expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already transformation in the absence of secretory changes. Hum Reprod. 2005;20:1541–1547. doi: 10.1093/humrep/deh793. [DOI] [PubMed] [Google Scholar]
- Friedler S, Gilboa S, Schachter M, Raziel A, Strassburger D, Ron ELR. Luteal phase characteristics following GnRH antagonist or agonist treatment – a comparative study. Reprod Biomed Online. 2006;12:27–32. doi: 10.1016/s1472-6483(10)60976-5. [DOI] [PubMed] [Google Scholar]