Abstract
Chimpanzees produce numerous species-atypical signals when raised in captivity. Here we report contextual elements of the use of two captivity-specific vocal signals, the “raspberry” and the extended grunt. Results demonstrate that these vocalizations are not elicited by the presence of food; rather the data suggest that these vocalizations function as attention-getting signals. These findings demonstrate a heretofore underappreciated category of animal signals: novel signals invented in novel environmental circumstances. The invention and use of species-atypical signals, considered in relation to group differences in signaling repertoires in apes in their natural habitats, may index a generative capacity in these hominoid species without obvious corollary in other primate species.
A heretofore little-studied, yet theoretically important class of animal signals are those produced uniquely within some, but not other populations of the same species. Nowak, Plotkin, and Jansen (2000) listed three putatively exhaustive categories of animal communication designs: “a finite repertoire of calls … ; a continuous analogue signal … ; and series of random variations on a theme” (p. 495). Clearly, the class of novel signals developed by animals is missing from that list. The number of such signals in both the auditory and visual domains is growing rapidly, with recent empirical research into the communicative repertoires of both captive and wild chimpanzees. Wild chimpanzees exhibit a number of group differences in their communicative repertoires, including parametric—as opposed to qualitative—differences in their vocal repertoires (e.g., Crockford, Herbinger, Vigilant, & Boesch 2004), and qualitative differences in their visual signals, including patchy distribution of the leaf-clipping display (Nishida, 1980) and hand-clasping while grooming (McGrew & Tutin 1978). There are captivity-specific attention-directing manual gestures (e.g., pointing with fingers, Leavens, Russell, & Hopkins 2005a), other attention-getting auditory signals such as hand clapping (e.g., Hostetter, Cantero, & Hopkins 2001; Leavens, Hostetter, Wesley, & Hopkins 2004a), and facial expressions that serve as iconic gestures (see, e.g., Figure 1 in Leavens & Hopkins 1998). Far from being scientifically irrelevant consequences of artificial, captive environments, these signals are being produced by chimpanzees who do not, presumably, differ systematically in their genetic complements from their wild counterparts (e.g., Leavens 2004; Leavens, Hopkins, & Bard 2005b). Common to all of these diverse signals is the complete absence of any overt attempt to train their development. Hence, chimpanzees generate novel signals in novel, artificial circumstances.
Figure 1.
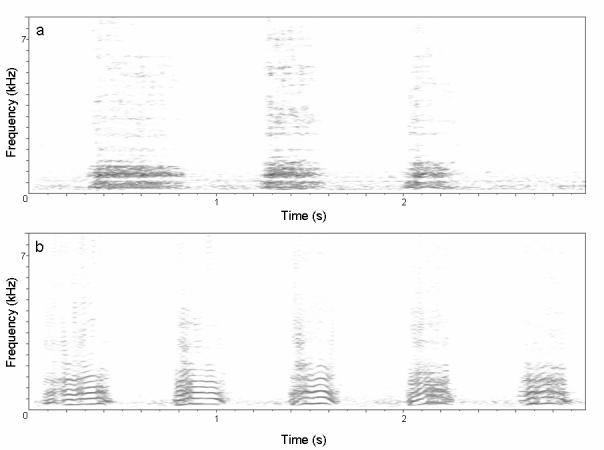
Figure 1a: Spectrograph of “extended grunt”
Figure 1b: Spectrograph of “raspberry” vocalization. Note that the apparent harmonic structure appears to be produced by labial, rather than vocal cord vibrations.
In contrast to the numerous studies on vocal communication in monkeys, very little research has focused on vocal communication in great apes. However, a few studies have reported that chimpanzees and bonobos produce context-specific vocalizations in a number of ecological, social, and behavioral situations (Crockford & Boesch 2003; Notman & Rendall 2005; Slocombe & Zuberbuhler 2005, Taglialatela, Savage-Rumbaugh, & Baker 2003). In addition, recent studies on captive chimpanzees have demonstrated that they intentionally produce manual gestures (Call & Tomasello 1994; Hostetter et al. 2001; Krause & Fouts 1997; Leavens et al. 2004a; Tomasello, Call, Nagell, Olguin, & Carpenter 1994). In these studies, food is typically placed outside the focal subject's home cage and is therefore out of reach from the subject. In these circumstances, chimpanzees and other great apes only gesture to the food when a human is present and visually oriented toward the subject (Call & Tomasello 1994; Hostetter et al. 2001; Krause & Fouts 1997; Leavens, Hopkins, & Bard 1996; Leavens, Hopkins, & Thomas 2004b; Tomasello et al. 1994). Moreover, chimpanzees alternate their gaze between the referent (food) and the social agent while gesturing (Leavens & Hopkins 1998) and “repair” their gestural communication when it has failed (Leavens et al. 2005a).
Consistent with these findings, several studies have claimed that captive chimpanzees (and other apes) produce vocalizations and other non-vocal acoustic signals, such as banging their cage or hand clapping, as a means of capturing the attention of an otherwise inattentive social agent (Hostetter et al. 2001; Leavens et al. 2004a; Tomasello et al. 1994). Here we report characteristics of the use of two such signals, an extended grunt and a raspberry (this latter also known as a “Bronx cheer” or a “splutter”). Both of these signals are common in captivity, but the raspberry has not, to our knowledge, been reported in any wild chimpanzee group (a similar sound has been reported in some, but not all groups of wild orangutans, Pongo pygmaeus, van Schaik, Ancrenaz, Borgen, Galdikas, Knott, Singleton, Suzuki, Utami, & Merrill, 2003). The raspberry is recognized by researchers to function as an attention-getting device (Leavens & Hopkins, unpublished data; Wrangham, personal communication), but has received very little experimental scrutiny (see Marshall, Arcadi, & Wrangham 1999 for a brief discussion of the raspberry).
The primary aim of this study was to determine if chimpanzees selectively produce the “raspberry” and “extended grunt” vocalizations, (herein referred to as attention-getting calls), to capture the attention of a human. To accomplish this goal, we compared the occurrence and frequency of attention-getting sounds and species-typical food vocalizations when either a) a human alone was positioned in front of the chimpanzees b) food was placed in front of the chimpanzees with no human present and c) food was placed in front of the chimpanzees and a human was standing in proximity to the food. Our hypothesis was that if the attention-getting sounds are used to capture the attention of the human then they should occur more frequently when a human and food are present together compared to either the human alone or food alone conditions. We further hypothesized that food calls would be produced more frequently when the food was presented alone compared to the human alone and human and food conditions.
Methods
Subjects
Twenty-four subjects, (15 females, 9 males) ranging in age from 9 to 42 years old were included in this study (Mean = 19.08, s.d. = 11.79). All subjects were housed at the Yerkes National Primate Research Center of Emory University and had been used in previous studies on gestural and vocal communication (see Hostetter et al. 2001; Leavens et al., 2004a; Leavens et al. 2005a). As the focus of this study was on how chimpanzees use the “raspberry” and “extended grunt” (see Figure 1), the 24 subjects were randomly selected from a larger cohort of chimpanzees (n = 55) that had previously been identified to vocalize in the presence of a human holding food (see Leavens et al. 2004a).
Procedure
Subjects were tested in their home cages and were tested either in isolation or in pairs. Each subject was tested once in three conditions including a) food alone (FA) b) human alone (HA) or c) human and food (HF). In the FA condition, an experimenter would place a whole banana on the ground outside the subject's home cage, 1 meter from the subject and would immediately leave the area. The human experimenter was familiar to all of the chimpanzees, and has worked regularly with and frequently provided food to all of the subjects for approximately the past 15 years. In the HA condition, the experimenter would position themselves approximately 2 meters in front of the subject. The human faced away from the focal subject and stood motionless during the duration of the trial (60 secs). In the HF condition, the experimenter would place a banana 1 meter from the subject and stand two meters from the focal subject with their back to the focal subject. Each trial lasted one minute and the order of presentation of the conditions was counterbalanced across subjects using the Latin square technique (8 subjects tested in each of three different orders). In the FA and HF conditions, the food was hidden from the subject and therefore could not be seen until at was placed on the ground in front of the focal subject. All subjects were tested on one day and approximately 30 seconds separated the trials for each condition. All trials were videotaped with a digital video camera positioned on a tripod 1 meter from the focal subject and scored later for the frequency of vocalizations in each condition. From the videotape, the experimenter recorded the number of attention-getting sounds, and the number of food calls made by each of the subjects in each of the conditions during each 1 minute trial. Occasionally the subjects pant-hooted during the trials but these were infrequent (n = 4) and therefore were not scored. Animals were not isolated from the rest of the colony during testing, as this practice introduces an element of artificiality into what is designed to be a fairly naturalistic procedure; i.e., a situation in which the chimpanzees in their familiar social groups are faced with unreachable food is consistent with the life experiences of these subjects, but social isolation is not a common experience for these animals and would, therefore, introduce an unnecessary confound into the procedure.
To assure reliability in measurement of the frequencies of occurrence of each vocal type, the videotapes were recoded one month after the initial data collection by the same observer who scored the original tapes. Tests from 10 randomly selected subjects were used to assess reliability. With respect to attention-getting calls, for the FA, HA and HF conditions, test-retest correlations were 1.0, .883 and 1.0, respectively. For the food calls, test-retest correlations for the FA, HA and HF conditions were 95, 1.0, and 1.0, respectively. Thus, intra-rater agreements in recording the frequency of different vocal types were significant and high in each experimental condition. We also performed inter-rater reliability. A second experimenter, who had not previously scored nor seen any of the previous data or tapes, scored 6 of the individuals for the frequency of attention-getting and food calls in each of the three conditions. For the attention-getting sounds, test-retest correlations for the FA, HA and HF conditions were 1.0, 1.0, and .99. For the food calls, test-retest correlations for the FA, HA and HF conditions were 1.0, 1.0, and .90, respectively
Results
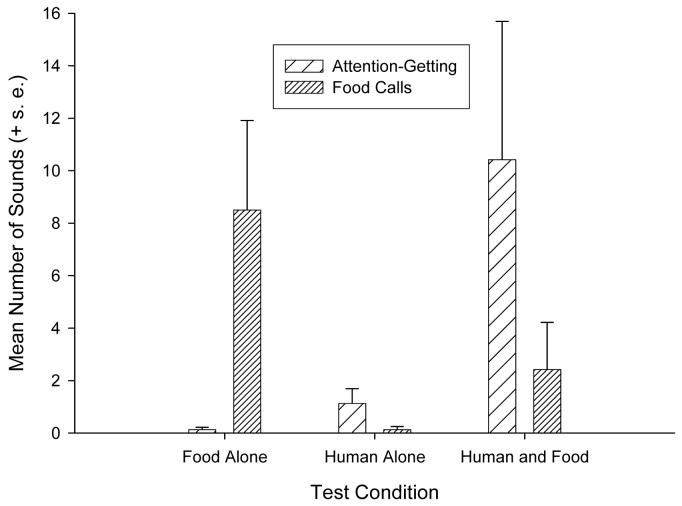
Shown in Table 1 are the total frequencies of each vocal type and experimental condition for all subjects. For both the attention-getting sounds X2(2, N=24) = 21.81, p < .01 and food calls X2(2, N=24) = 13.77, p < .01, Friedman tests indicated significant differences between the three conditions. The mean number of attention-getting sounds and food calls produced in each condition are shown in Figure 2. Subsequent post-hoc tests using Wilcoxon tests indicated that the number of attention-getting sounds produced in the HF condition was significantly higher than in the HA (z = 2.75, p < .05) and FA (z = 3.63, p < .01) conditions. Similarly, post-hoc tests indicated that the number of food calls produced in the FA condition was significantly higher than in the HA (z = 2.81, p < .01) and HF conditions (z = 2.09, p < .05).
Table 1.
Individual Data for Each Test Condition and Vocalization
| Attention-Getting | Food Calls | |||||
|---|---|---|---|---|---|---|
| Subject | HA | FA | HF | HA | FA | HF |
| Females | ||||||
| Anja | 0 | 1 | 15 | 0 | 0 | 0 |
| Beleka | 0 | 0 | 6 | 0 | 0 | 0 |
| Brandy | 2 | 0 | 4 | 0 | 58 | 3 |
| Cathy | 0 | 0 | 2 | 0 | 0 | 0 |
| Cheeta | 2 | 0 | 0 | 0 | 2 | 1 |
| Dara | 0 | 0 | 29 | 0 | 2 | 0 |
| Edwina | 2 | 0 | 6 | 0 | 0 | 0 |
| Elvira | 0 | 2 | 128 | 0 | 0 | 0 |
| Faye | 0 | 0 | 0 | 0 | 30 | 0 |
| Leslie | 0 | 0 | 0 | 0 | 0 | 0 |
| Lilone | 0 | 0 | 5 | 0 | 0 | 0 |
| Liza | 0 | 0 | 0 | 0 | 0 | 0 |
| Mai | 0 | 0 | 1 | 0 | 0 | 0 |
| Melissa | 0 | 0 | 10 | 0 | 0 | 0 |
| Sylvia | 3 | 0 | 3 | 0 | 0 | 0 |
| Males | ||||||
| Elwood | 0 | 0 | 0 | 0 | 0 | 0 |
| Fritz | 1 | 0 | 6 | 0 | 2 | 6 |
| Iyk | 0 | 0 | 1 | 0 | 0 | 0 |
| Jarred | 0 | 0 | 10 | 0 | 19 | 0 |
| Jolson | 0 | 0 | 4 | 0 | 38 | 43 |
| Joseph | 0 | 0 | 4 | 0 | 8 | 0 |
| Les | 0 | 0 | 0 | 0 | 8 | 0 |
| Patrick | 13 | 0 | 8 | 3 | 45 | 5 |
| Winston | 4 | 0 | 0 | 0 | 0 | 0 |
Figure 2.
Mean (+ s.e.) number of food calls and attention-getting sounds as a function of whether food was presented alone, a human was present alone or a human and food were presented at the same time.
In terms of individual variation in the vocal production between the three conditions, 71% of the chimpanzees (17/24) produced at least one attention-getting call in the HF condition compared to only 29% in the HA and 8% in the FA conditions. McNemar tests indicated that the proportion of subjects producing a attention-getting sound in the HF conditions was significantly higher than in the HA (z = −2.67, p < .02) and FA (z = −3.87, p < .001) conditions. There was no significant difference in the proportion of subjects producing attention-getting sounds in the FA and HA conditions. For the food calls, 42% of the chimpanzees made a food call in the FA condition compared to 21% in the HF and 4% in the HA conditions. Similarly, McNemar tests indicated that the number of individuals producing food calls in the FA condition was significantly higher than in the HA condition (z = −3.00, p < .01). No other differences were significant.
In the next analysis, we compared the frequencies in the production of attention-getting and food calls across the three test conditions as a function of whether the chimpanzees were tested alone (n = 9) or with a cagemate (n = 15). This was done to assess whether the observed pattern of results might be due to the presence of an audience or emotional contagion. For each type of sound, a mixed model ANOVA was performed with condition (FA, HA, HF) serving as the repeated measure while housing condition (alone, with cagemate) was the between-group factor. For both food calls and attention-getting sounds, no significant main effects or interactions were found. The mean number of attention-getting sounds and food calls in the FA, HA and HF conditions as a function of the housing condition are shown in Table 2.
Table 2.
Mean Number of Attention-Getting and Food Calls As a Function of the Housing Conditions of the Chimpanzees
| Attention-Getting | Food Calls | |||||
|---|---|---|---|---|---|---|
| HA | FA | HF | HA | FA | HF | |
| Housing Condition | ||||||
| Alone | .67 | .01 | 6.44 | .01 | 15.00 | 5.22 |
| With Cage Mate | 1.40 | .20 | 4.13 | .20 | 5.13 | .73 |
Lastly, to test whether the observed effects were specific to “raspberries” or “extended grunts”, we compared the frequencies in vocalization for each of the three conditions as a function of whether the chimpanzees made “raspberries” (n = 17) or “extended grunts” (n = 7) using a mixed model analysis of variance. Test condition was the repeated measure whereas vocal type was the between-group factor. No significant main effects or interactions with the vocal type variable were found in these analyses.
Discussion
In this study, we sought to determine if chimpanzees selectively produce specific vocalizations in order to capture the attention of a human. Chimpanzee vocal production and frequency was compared in three experimental conditions: 1) the presence of a human alone, 2) the presence of food alone, and 3) the presence of a human and food. Both the type of vocalization as well as the number of occurrences was recorded. The results indicated that chimpanzees were more likely to produce two specific calls, (the “raspberry,” and the “extended grunt” – so-called, “attention-getting sounds”) when a human was present in conjunction with a preferred food item, than they were in either of the two other conditions. In addition, chimpanzees were more likely to produce traditionally defined “food” vocalizations in the presence of food alone as they were when food was presented in conjunction with a human, or when a human was alone. These results suggest that chimpanzees are using context-specific vocalizations and may intentionally produce these sounds.
These data indicate that captive chimpanzees alter the type of vocalizations they produce depending on the context and communicative demands of the situation. In other words, when chimpanzees need to capture the attention of an otherwise inattentive human, they produce “raspberry” or “extended grunt” vocalizations and rarely produce food calls. In contrast, upon sight of food by itself, chimpanzees produce significantly more food calls compared to when a human is present alone or present in conjunction with the food. In other words, attention-getting sounds are used as a means of manipulating the attentional status of a human, whereas food calls appear to be associated with the presence of food. Reinforcing the view that the raspberry and extended grunt are used as attention-getting sounds is the evidence that they are also produced to capture the attention of a human facing away from them who is holding a tool needed for a problem solving task (Russell, Braccini, Buehler, Kachin, Schapiro, & Hopkins, 2005). Thus, the presence of food in conjunction with a human is not the only condition under which raspberries and extended grunts are produced. Collectively, these results suggest that the chimpanzees may produce these sounds intentionally. It is interesting that none of the chimpanzee subjects produced both “raspberry” and “extended grunt” attention getting vocalizations. One possible explanation for this observation is that those individuals that have acquired one of these two so-called attention getting sounds do not need to produce a second type in order to achieve the intended result. In other words, producing a “raspberry” may be just as effective as producing an “extended grunt” at acquiring the attention of a human experimenter. In this case, the chimpanzees may need only incorporate one of these sounds into their repertoire. Certainly, this would speak to the functional equivalence of the two sounds. However, at the YNPRC there are chimpanzees that produce both the “raspberry” and “extended grunt.” We plan to study the use of these vocalizations more closely in the future.
The results reported here differ from previous studies that have documented audience effects in chimpanzees and other primates. In previous studies on chimpanzees, changes in the rates of production of specific vocalizations were altered by parameters associated with either food quantity or divisibility (Hauser, Teixidor, Field, & Flaherty 1993) as well as the absence or presence of a social partner (Brosnan & de Waal 2003). In contrast, rather than alter the calling rate of a specific vocalization in response to the presence or absence of an audience, the results reported here indicate the chimpanzees produce different vocalizations depending on the presence or absence of a human in association with a food item. This suggests a functional distinction in the chimpanzees' use of vocalizations based on the communicative context. Although it is not clear if the chimpanzees are silencing their food vocalizations in the HF condition in favor of attention getting calls, the data clearly indicate that the chimpanzees are controlling what sounds they produce. Moreover, the fact that the “raspberry” is not part of the species-typical repertoire of sounds in chimpanzees further supports the view this sound has been individually learned by the animals, and that their production may involve a volitional component. Of course it remains possible that these sounds do exist in wild populations, but have not yet been observed.
It remains possible that these putative “attention-getting” vocalizations are elicited by the presence of people (as opposed to the apparent attentional status of people), irrespective of the other factors manipulated here, but we believe this is unlikely for two reasons. First, very few such signals were displayed in the Human Alone condition (see Figure 2); it was, thus, the combined presentation of humans and food that elicited the majority of these particular vocalizations. In fact, the occurrence of “attention-getting” sounds in the HA condition were entirely attributable to being preceeded by a HF trial in the three trial sequence. Thus, there have been some carry over from the HF to the HA condition because a human had previously been present with a food. Secondly, we know from previous research that chimpanzees are sensitive to the apparent attentional status of a communicative partner when communicative partners are conspecifics (Tomasello et al. 1994) and when the communicative partners are humans (e.g., Hostetter et al. 2004; Leavens et al. 2004a).
In our view, the best framework for the proximate interpretation of the current findings as well as previous results on gestural communication in captive chimpanzees is that the apes are engaging in a form of social tool use in response to what has been referred to as the “referential problem space” (Leavens et al.2005a). Tool use has been defined as, “the external employment of an unattached environmental object to alter more efficiently the form, position, or condition of another object, another organism, or the user itself when the user holds or carries the tool during or just prior to use and is responsible for the proper and effective orientation of the tool” (Beck, 1980). Wild and captive chimpanzees are well known for their tool using abilities, and the results of our studies meet this definition. Within this framework, the chimpanzees have a goal – to obtain a food item that they cannot otherwise attain. In this context, the chimpanzees utilize their communicative behaviors to manipulate a human (the tool) to attain a food item (goal). Moreover, their communicative behavior is flexible in this context because the chimpanzees alter the modality of their communicative signals in accordance with the attentional state of the human. Specifically, when the human is facing them, they are more likely to use visual signals such as manual gestures but will use auditory signals, including vocalizations, when the human is facing away (Hostetter et al. 2001; Leavens et al. 2004a). Thus, both chimpanzees and humans can tactically deploy their communicative signals towards immediate ends using, presumably homologous problem-solving capacities (e.g., Leavens et al. 2005a,b).
The ultimate significance of these findings is that these vocalizations represent evidence for the invention and use of novel signals in a nonhuman animal, in instrumental contexts. The generative nature of chimpanzee communication has significance for our understanding of language evolution. This generative quality is frequently listed as a trait uniquely characteristic of human languages (e.g., Hauser, Chomsky, & Fitch 2004), a claim that we believe is refuted by the diverse array of signals developed and used by chimpanzees to communicate with their human social partners. This creative potential displayed by chimpanzees evokes a mode of communication that may have relatively limited distribution among vertebrates. This mode is characterized by a persistent thrusting and parrying of signals, indicative of a strong motivation to co-create meaning, what Tomasello (e.g., Tomasello & Call 1997) refers to as “ontogenetic ritualization.” Whereas ontogenetic ritualization strictly denotes a process of signal acquisition, we suggest that there might be considerable variability across primate species in their motivation to engage in this process (e.g., Bard,1998). Clearly, other primates can discriminate and adaptively use ecologically relevant signals (e.g., Zuberbuhler 2000), but what may distinguish the great apes from other anthropoids is the motivation for this creative, or generative signal development. Chimpanzee signals lack the recursive qualities that truly set human languages apart (cf. Hauser et al. 2004), nevertheless, as far as we know, few other primates invent as wide a range of signals in such diverse modalities as do chimpanzees (cf. Tomasello, George, Kruger, Farrar, & Evans 1985, Tomasello et al. 1994), although this claim must be considered tentative in the absence of extensive comparative research into population-specific communicative signals. This multimodal generative capacity is implicit in several recent models of language evolution (e.g., Hauser et al. 2004; Nowak et al. 2000) and becomes especially evident when chimpanzees are raised in captive environments. Thus, the capacity not merely to discriminate, but to generate novel communicative signals, which is a hallmark of human linguistic communication, may have relatively ancient roots in the signaling characteristics of our ape ancestors.
Acknowledgement
This work was supported in part by NIH grants RR-00165, NS-36605, and NS-42867.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Bard KA. Social-experiential contributions to imitation and emotion in chimpanzees. In: Bråten S, editor. Intersubjective communication and emotion in early ontogeny: A source book. Cambridge University Press; Cambridge, UK: 1998. pp. 208–227. [Google Scholar]
- Beck B. Animal Tool Behavior. The Use and Manufacture of Tools by Animals. Garland STPM; New York: 1980. [Google Scholar]
- Brosnan SF, de Waal FBM. Regulation of vocal output by chimpanzees finding food in the presence or absence of an audience. Evolution of Communication. 2003;4(2):211–224. [Google Scholar]
- Call J, Tomasello M. Production and comprehension of referential pointing by orangutans (Pongo pygmaeus) Journal of Comparative Psychology. 1994;108:307–317. doi: 10.1037/0735-7036.108.4.307. [DOI] [PubMed] [Google Scholar]
- Crockford C, Herbinger I, Vigilant L, Boesch C. Wild chimpanzees produce group-specific calls: A case for vocal learning? Ethology. 2004;110:221–243. [Google Scholar]
- Fitch TW. The evolution of speech: A comparative review. Trends in Cognitive Science. 2000;4:258–267. doi: 10.1016/s1364-6613(00)01494-7. [DOI] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe: Patterns in Adaptation. Harvard University Press; Cambridge, MA: 1986. [Google Scholar]
- Hauser MD, Teixidor P, Field L, Flaherty R. Food-elicited calls in chimpanzees: Effects of food quantity & divisibility. Animal Behaviour. 1993;45:817–819. [Google Scholar]
- Hauser MD. Functional referents and acoustic similarity: Field playback experiments with rhesus monkeys. Animal Behaviour. 1998;55:1647–1658. doi: 10.1006/anbe.1997.0712. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT. The faculty of language: What is it, who has it, and how did it evolve? Science. 2004 November 22;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- Hostetter AB, Cantero M, Hopkins WD. Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens) Journal of Comparative Psychology. 2001;115:337–343. doi: 10.1037//0735-7036.115.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA. Manual deixis in apes and humans. Interaction Studies. 2004;5:387–408. [Google Scholar]
- Leavens DA, Hopkins WD. Intentional communication by chimpanzees: A cross-sectional study of the use of referential gestures. Developmental Psychology. 1998;34:813–822. doi: 10.1037//0012-1649.34.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Indexical and referential pointing in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1996;110:346–353. doi: 10.1037/0735-7036.110.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Thomas RK. Referential communication by chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 2004b;118:48–57. doi: 10.1037/0735-7036.118.1.48. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Understanding the point of chimpanzee pointing: Epigenesis and ecological validity. Current Directions in Psychological Science. 2005b;14:185–189. doi: 10.1111/j.0963-7214.2005.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Russell JL, Hopkins WD. Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes) Child Development. 2005a;76:291–306. doi: 10.1111/j.1467-8624.2005.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Animal Behaviour. 2004a;67:467–476. [Google Scholar]
- Marshall AJ, Wrangham RW, Arcadi AC. Does learning affect the structure of vocalizations in chimpanzees? Animal Behaviour. 1999;58:825–830. doi: 10.1006/anbe.1999.1219. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Tutin CEG. Evidence for a social custom in wild chimpanzees? Man. 1978;13:243–251. [Google Scholar]
- Nishida T. The leaf-clipping display: A newly-discovered expressive gesture in wild chimpanzees. Journal of Human Evolution. 1980;9:117–128. [Google Scholar]
- Notman H, Rendall D. Contextual variation in chimpanzee pant hoots and its implications for referential communication. Animal Behaviour. 2005;70:177–190. [Google Scholar]
- Nowak MA, Plotkin JB, Jansen VA. The evolution of syntactic communication. Nature. 2000 March 30;404:495–498. doi: 10.1038/35006635. [DOI] [PubMed] [Google Scholar]
- Premack D. Is language the key to human intelligence? Science. 2004;303:318–320. doi: 10.1126/science.1093993. [DOI] [PubMed] [Google Scholar]
- Randolph MC, Brooks C. Conditioning of a vocal response through social reinforcement. Folia Primatologica. 1967;5:70–79. doi: 10.1159/000161938. [DOI] [PubMed] [Google Scholar]
- Russell J, Braccini S, Buehler N, Kachin M, Schapiro SJ, Hopkins WD. Chimpanzees (Pan troglodytes) intentional communication is not contingent upon food. Animal Cognition. 2005;8:263–272. doi: 10.1007/s10071-005-0253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL. Behavioral mechanisms underlying vocal communication in nonhuman primates. Animal Learning & Behavior. 1997;25:249–267. [Google Scholar]
- Seyfarth RM, Cheney DL, Marler P. Vervet monkey alarm calls: Semantic communication in a free-ranging primate. Animal Behaviour. 1980;28:1070–1094. [Google Scholar]
- Slocombe KE, Zuberbühler K. Agonistic screams in wild chimpanzees (Pan troglodytes schweinfurthii) vary as a function of social role. Journal of Comparative Psychology. 2005;119:67–77. doi: 10.1037/0735-7036.119.1.67. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. Auditory communication among vervet monkeys (Cercopithecus aethiops) In: Altmann SA, editor. Social communication among primates. University of Chicago Press; Chicago: 1967. pp. 281–384. [Google Scholar]
- Taglialatela JP, Savage-Rumbaugh S, Baker LA. Vocal production by a language-competent Pan paniscus. International Journal of Primatology. 2003;24:1–17. [Google Scholar]
- Tomasello M, Call J. Primate cognition. Oxford University Press; Oxford: 1997. [Google Scholar]
- Tomasello M, George BL, Kruger AC, Farrar MJ, Evans A. The development of gestural communication in young chimpanzees. Journal of Human Evolution. 1985;14:175–186. [Google Scholar]
- Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. The learning and use of gestural signals by young chimpanzees: A trans-generational study. Primates. 1994;35:137–154. [Google Scholar]
- van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. Orangutan cultures and the evolution of material culture. Science. 2003 January 3;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Vea JJ, Sabater-Pi J. Spontaneous pointing behaviour in wild pygmy chimpanzees (Pan paniscus) Folia Primatologica. 1998;69:289–290. doi: 10.1159/000021640. [DOI] [PubMed] [Google Scholar]
- Zuberbuhler K. Interspecies semantic communication in two forest primates. Proceedings of the Royal Society of London, Series B. 2000;267:213–218. doi: 10.1098/rspb.2000.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbuhler K, Cheney DL, Seyfarth RM. Conceptual semantics in a nonhuman primate. Journal of Comparative Psychology. 1999;113:33–42. [Google Scholar]