Abstract
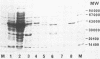
An aminotransferase capable of transaminating 2-oxo-4-[(hydroxy)(methyl)phosphinoyl]butyric acid to L-phosphinothricin [L-homoalanine-4-yl-(methyl)phosphinic acid], the active ingredient of the herbicide Basta (Hoechst AG), was purified to apparent homogeneity from Escherichia coli K-12. The enzyme catalyzes the transamination of L-phosphinothricin and various analogs with 2-ketoglutarate as the amino group acceptor. The transaminase has a molecular mass of 43 kilodaltons by sodium dodecyl sulfate-gel analysis and an isoelectric point of 4.35. The enzyme was most active in the high-pH region, with a maximum at pH 8.0 to 9.5, and had a temperature optimum of 55 degrees C. Heat stability was observed up to 70 degrees C. Substrate specificity studies suggested that the enzyme is identical with the 4-aminobutyrate:2-ketoglutarate transaminase (EC 2.6.1.19). The first 30 amino acids of the N terminus of the protein were determined by gas phase sequencing. The transaminase was immobilized by coupling to the epoxy-activated carrier VA-Biosynth (Riedel de Haen) and used in a column reactor for the continuous production of L-phosphinothricin. The enzyme reactor was operated for 7 weeks with only a slight loss of catalytic capacity. Production rates of more than 50 g of L-phosphinothricin per liter of column per h were obtained.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch K., Dichmann R., Schmitt P., Uhlmann E., Schulz A. Stereospecific production of the herbicide phosphinothricin (glufosinate) by transamination: cloning, characterization, and overexpression of the gene encoding a phosphinothricin-specific transaminase from Escherichia coli. Appl Environ Microbiol. 1990 Jan;56(1):7–12. doi: 10.1128/aem.56.1.7-12.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch K., Tebbe C. C. Initial steps in the degradation of phosphinothricin (glufosinate) by soil bacteria. Appl Environ Microbiol. 1989 Mar;55(3):711–716. doi: 10.1128/aem.55.3.711-716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E., Gugel K. H., Hägele K., Hagenmaier H., Jessipow S., König W. A., Zähner H. Stoffwechselprodukte von Mikroorganismen. 98. Phosphinothricin und Phosphinothricyl-Alanyl-Alanin. Helv Chim Acta. 1972 Jan 31;55(1):224–239. doi: 10.1002/hlca.19720550126. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnett G., Yonaha K., Toyama S., Soda K., Walsh C. Studies on the kinetics and stoichiometry of inactivation of Pseudomonas omega-amino acid:pyruvate transaminase by gabaculine. J Biol Chem. 1980 Jan 25;255(2):428–432. [PubMed] [Google Scholar]
- Dover S., Halpern Y. S. Utilization of -aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol. 1972 Feb;109(2):835–843. doi: 10.1128/jb.109.2.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Coggins J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem J. 1986 Feb 15;234(1):49–57. doi: 10.1042/bj2340049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham I. G., Dacey S. A., Taylor P. P., Smith T. J., Hunter M. G., Finlay M. E., Primrose S. B., Parker D. M., Edwards R. M. The cloning and sequence analysis of the aspC and tyrB genes from Escherichia coli K12. Comparison of the primary structures of the aspartate aminotransferase and aromatic aminotransferase of E. coli with those of the pig aspartate aminotransferase isoenzymes. Biochem J. 1986 Mar 15;234(3):593–604. doi: 10.1042/bj2340593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia V., Carlomagno M. S., Nappo A. G., Bruni C. B. Cloning, structure, and expression of the Escherichia coli K-12 hisC gene. J Bacteriol. 1985 Dec;164(3):1317–1323. doi: 10.1128/jb.164.3.1317-1323.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu S., Ogawa T., Ogawa H., Kagamiyama H. Branched-chain amino acid aminotransferase of Escherichia coli: nucleotide sequence of the ilvE gene and the deduced amino acid sequence. J Biochem. 1985 Apr;97(4):993–999. doi: 10.1093/oxfordjournals.jbchem.a135176. [DOI] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando R. R., Bangerter F. W. The irreversible inhibition of mouse brain gamma-aminobutyric acid (GABA)-alpha-ketoglutaric acid transaminase by gabaculine. J Am Chem Soc. 1976 Oct 13;98(21):6762–6764. doi: 10.1021/ja00437a090. [DOI] [PubMed] [Google Scholar]
- Voellym R., Leisinger T. Role of 4-aminobutyrate aminotransferase in the arginine metabolism of Pseudomonas aeruginosa. J Bacteriol. 1976 Dec;128(3):722–729. doi: 10.1128/jb.128.3.722-729.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonaha K., Toyama S. gamma-Aminobutyrate:alpha-ketoglutarate aminotransferase from Pseudomonas sp. F-126: purification, crystallization, and enzymologic properties. Arch Biochem Biophys. 1980 Mar;200(1):156–164. doi: 10.1016/0003-9861(80)90342-2. [DOI] [PubMed] [Google Scholar]