Abstract
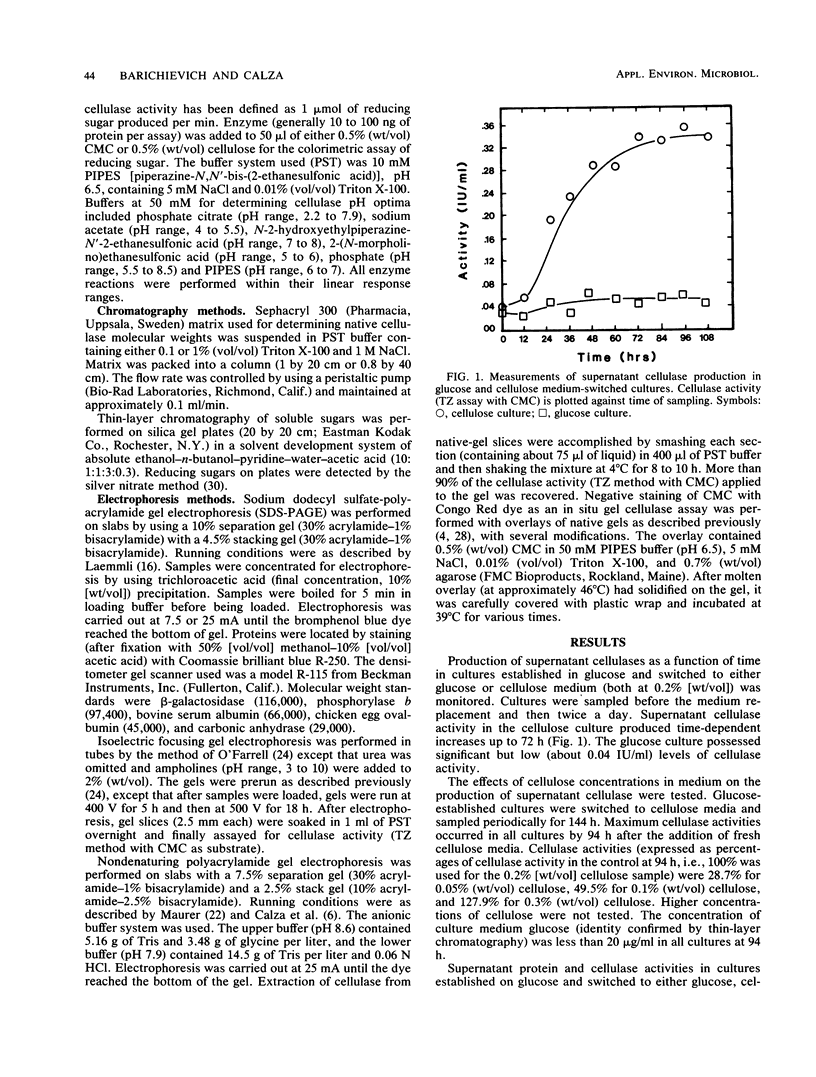
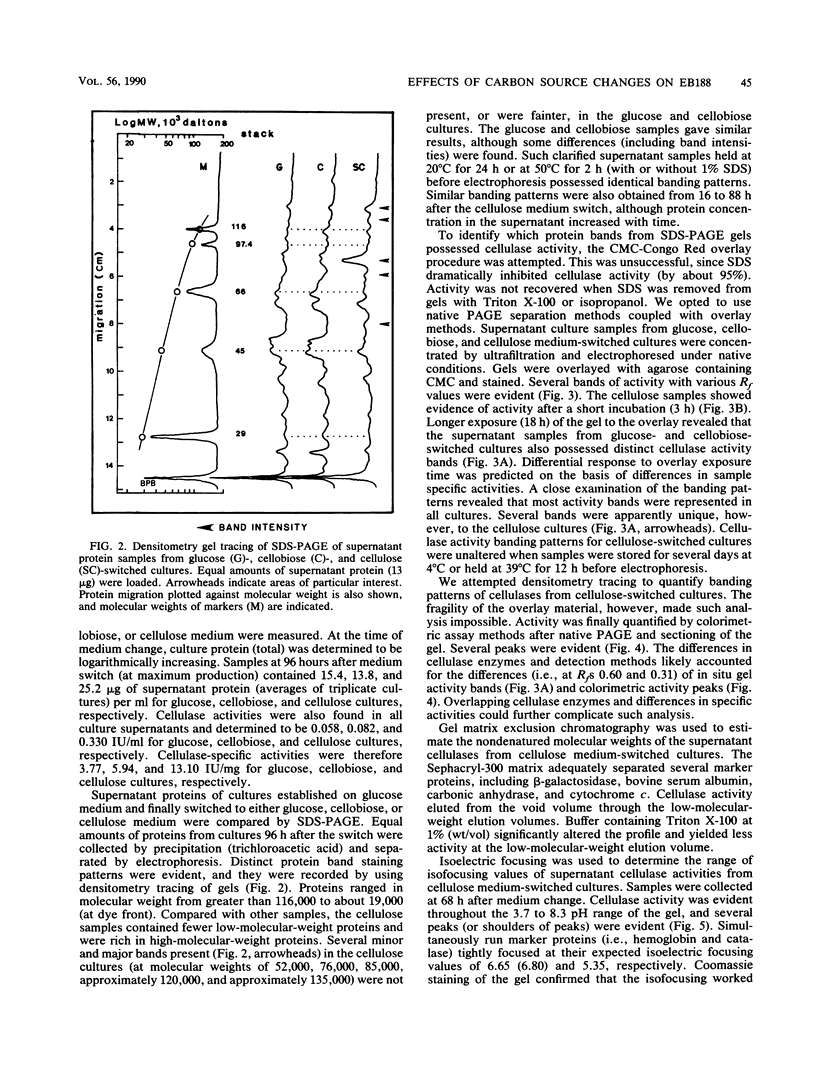
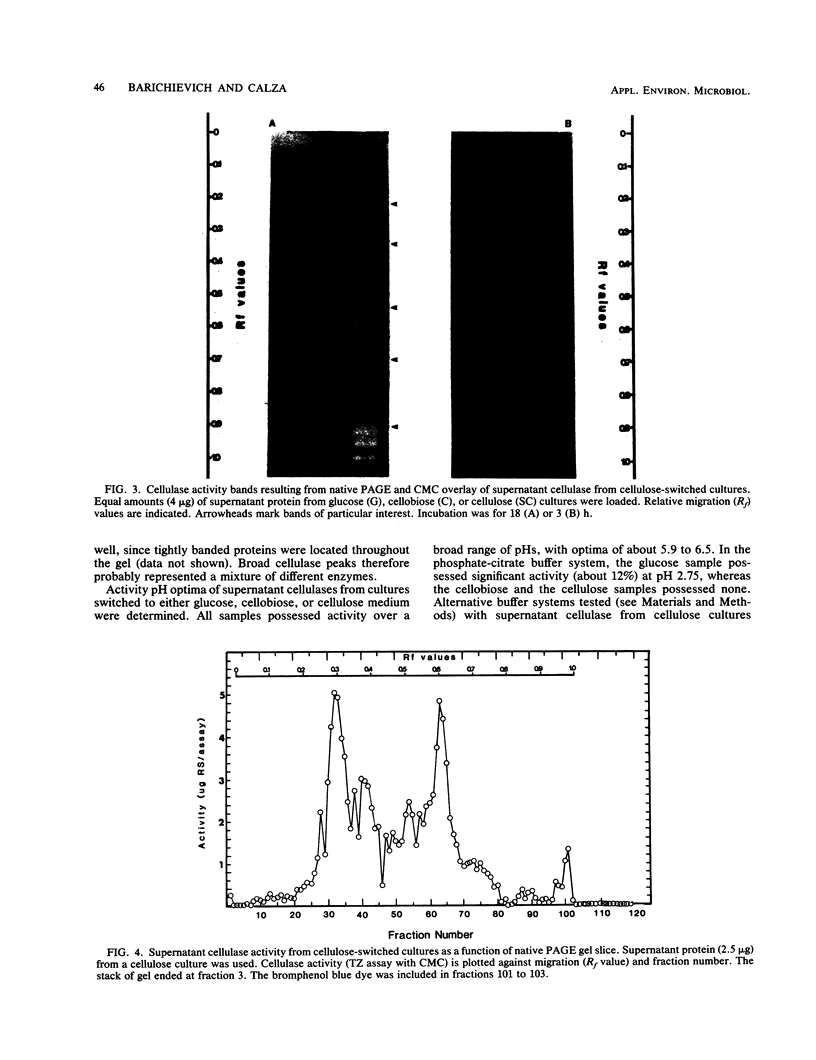
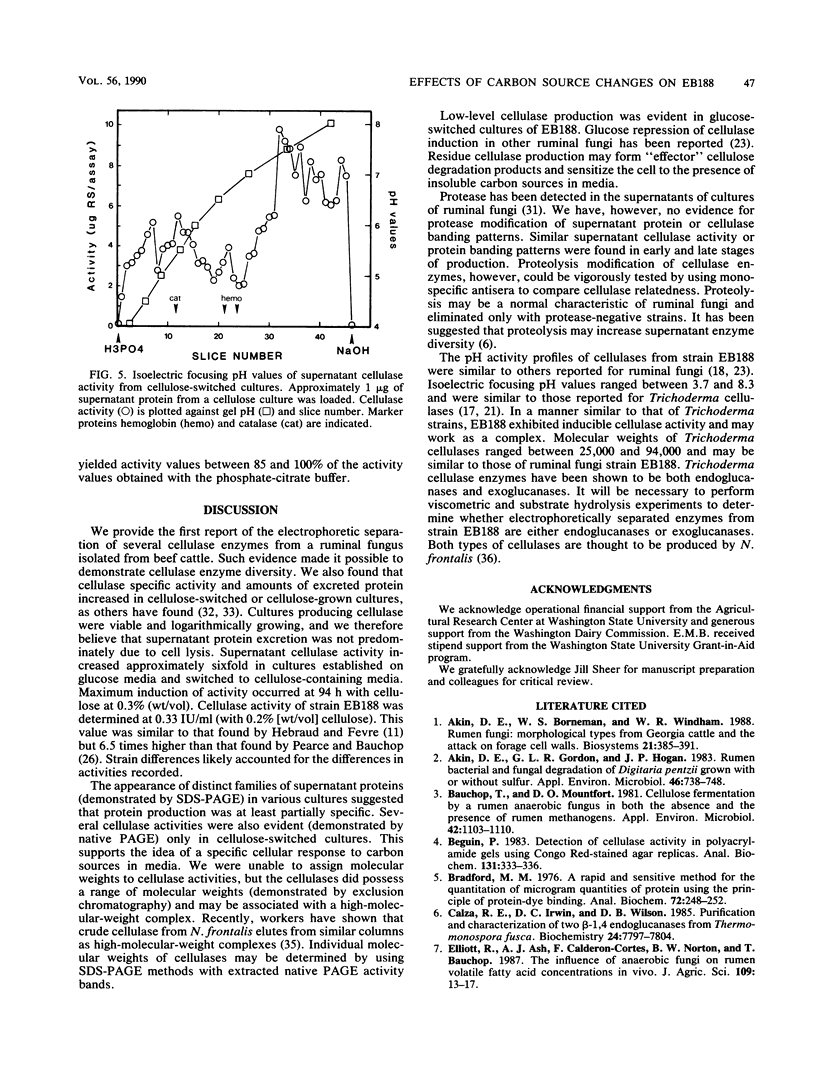
Protein and cellulose activities were measured in culture supernatants of the anaerobic ruminal fungus Neocallimastix frontalis EB188 established in glucose medium and switched to either glucose, cellobiose, or cellulose media. Polyacrylamide gel electrophoresis was used to show differences caused by changing medium carbon source. Culture supernatants contained proteins with molecular weights ranging from greater than 116,000 to about 19,000. Low levels of cellulose activity were evident in glucose-grown cultures. Increased amounts of slowly migrating cellulase activities appeared in the supernatants of glucose-grown cultures switched to cellulose. Cellulase activities which reacted differentially during colorimetric and in situ assays were produced. Isoelectric points of cellulase activities varied from 3.7 to 8.3, and activities possessed optimal pHs of between 5.9 and 6.5.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Borneman W. S., Windham W. R. Rumen fungi: morphological types from Georgia cattle and the attack on forage cell walls. Biosystems. 1988;21(3-4):385–391. doi: 10.1016/0303-2647(88)90037-8. [DOI] [PubMed] [Google Scholar]
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Gold J. J., Heath I. B., Bauchop T. Ultrastructural description of a new chytrid genus of caecum anaerobe, Caecomyces equi gen. nov., sp. nov., assigned to the Neocallimasticaceae. Biosystems. 1988;21(3-4):403–415. doi: 10.1016/0303-2647(88)90039-1. [DOI] [PubMed] [Google Scholar]
- Joblin K. N. Isolation, enumeration, and maintenance of rumen anaerobic fungi in roll tubes. Appl Environ Microbiol. 1981 Dec;42(6):1119–1122. doi: 10.1128/aem.42.6.1119-1122.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue C. K., Lipke P. N. Determination of reducing sugars in the nanomole range with tetrazolium blue. J Biochem Biophys Methods. 1985 Aug;11(2-3):109–115. doi: 10.1016/0165-022x(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Growth and fermentation of an anaerobic rumen fungus on various carbon sources and effect of temperature on development. Appl Environ Microbiol. 1987 Jun;53(6):1210–1215. doi: 10.1128/aem.53.6.1210-1215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandels M. Microbial sources of cellulase. Biotechnol Bioeng Symp. 1975;(5):81–105. [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. W., Gordon G. L. Sugar and polysaccharide fermentation by rumen anaerobic fungi from Australia, Britain and New Zealand. Biosystems. 1988;21(3-4):377–383. doi: 10.1016/0303-2647(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou M. K., Lowe S. E., Trinci A. P. The fermentative characteristics of anaerobic rumen fungi. Biosystems. 1988;21(3-4):371–376. doi: 10.1016/0303-2647(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Glycoside hydrolase enzymes present in the zoospore and vegetative growth stages of the rumen fungi Neocallimastix patriciarum, Piromonas communis, and an unidentified isolate, grown on a range of carbohydrates. Can J Microbiol. 1987 May;33(5):427–434. doi: 10.1139/m87-072. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]
- Windham W. R., Akin D. E. Rumen fungi and forage fiber degradation. Appl Environ Microbiol. 1984 Sep;48(3):473–476. doi: 10.1128/aem.48.3.473-476.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




