Abstract
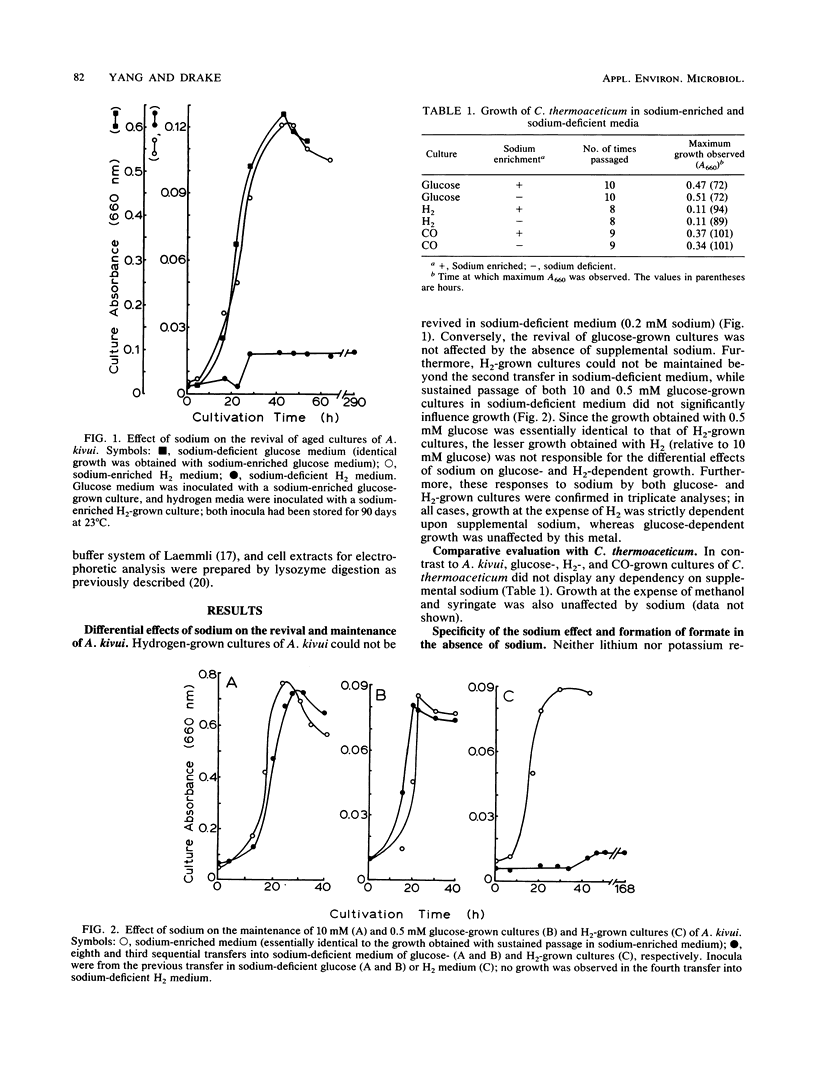
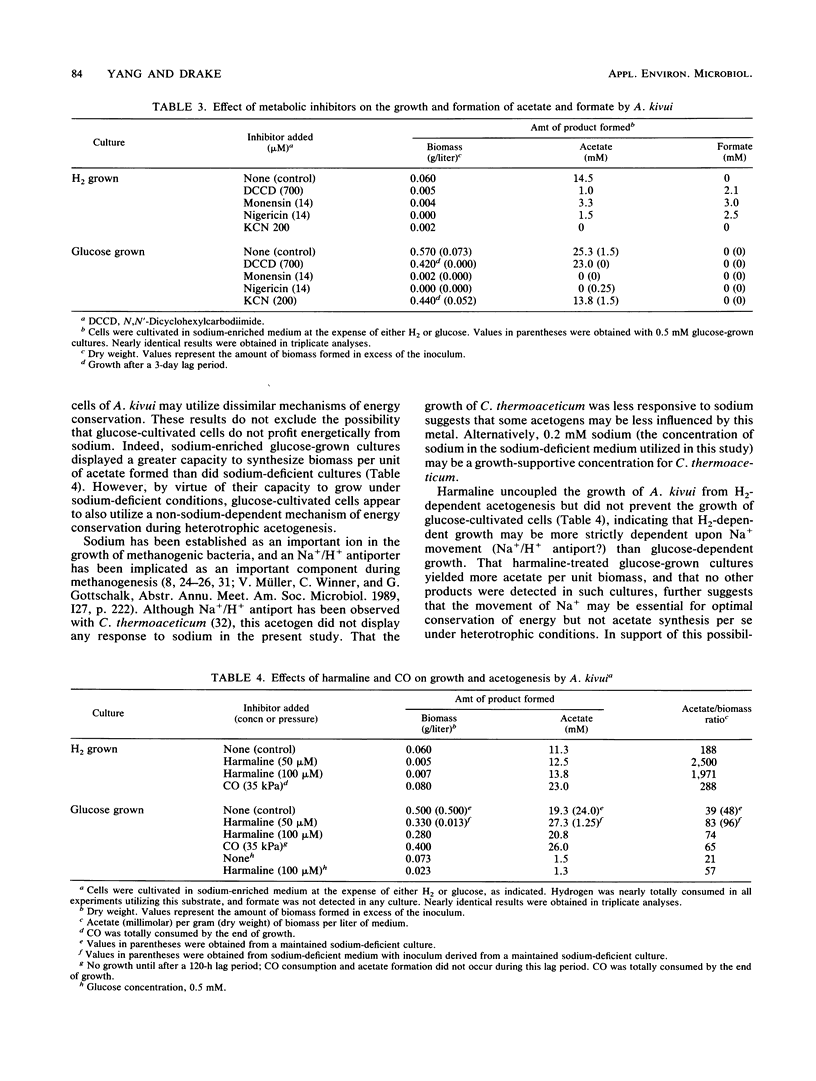
Acetogenium kivui could not be revived or maintained in a sodium-deficient medium (0.2 mM sodium) under H2-dependent conditions, and neither lithium nor potassium replaced the sodium requirement of H2-cultivated cells. Conversely, the revival and maintenance of glucose-cultivated cells did not display a dependency on supplemental sodium. In the absence of growth, formate became a major end product in both sodium-deficient and metabolically impaired H2-grown cultures of A. kivui. Harmaline, a putative inhibitor of Na+/H+ antiporters, uncoupled acetogenesis from H2-dependent growth but was less effective when growth was at the expense of glucose. Significantly, carbon monoxide (CO) stimulated H2-dependent growth of A. kivui but inhibited glucose-dependent growth. Collectively, these findings demonstrate that sodium plays a critical role in the H2-dependent bioenergetics of A. kivui and indicate that autotrophic and heterotrophic cells may utilize dissimilar mechanisms of energy conservation. In contrast to the growth of A. kivui, supplemental sodium was not required for the glucose-, H2-, and CO-dependent growth of Clostridium thermoaceticum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Bounds S. E. Harmaline inhibition of Na-dependent transport in renal microvillus membrane vesicles. Am J Physiol. 1980 Mar;238(3):F210–F217. doi: 10.1152/ajprenal.1980.238.3.F210. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Johnson R. L., Liu Y. Diffusion of the Interspecies Electron Carriers H(2) and Formate in Methanogenic Ecosystems and Its Implications in the Measurement of K(m) for H(2) or Formate Uptake. Appl Environ Microbiol. 1989 Jul;55(7):1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs G., Schönheit P., Diekert G. Sodium dependent acetate formation from CO2 in Peptostreptococcus products (strain Marburg). FEMS Microbiol Lett. 1989 Feb;57(3):253–257. doi: 10.1016/0378-1097(89)90309-1. [DOI] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise R., Müller V., Gottschalk G. Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol. 1989 Oct;171(10):5473–5478. doi: 10.1128/jb.171.10.5473-5478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ivey D. M., Ljungdahl L. G. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J Bacteriol. 1987 Dec;169(12):5845–5847. doi: 10.1128/jb.169.12.5845-5847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Ljungdahl L. G. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoautotrophicum. J Bacteriol. 1989 May;171(5):2873–2875. doi: 10.1128/jb.171.5.2873-2875.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey D. M., Ljungdahl L. G. Purification and characterization of the F1-ATPase from Clostridium thermoaceticum. J Bacteriol. 1986 Jan;165(1):252–257. doi: 10.1128/jb.165.1.252-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Drake H. L. Effects of cultivation gas phase on hydrogenase of the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Oct;160(1):466–469. doi: 10.1128/jb.160.1.466-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Drake H. L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Aug;159(2):700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Yang H. C., Heinonen J. K., Dean S. I., Drake H. L. Energy-dependent, high-affinity transport of nickel by the acetogen Clostridium thermoaceticum. J Bacteriol. 1988 Dec;170(12):5705–5708. doi: 10.1128/jb.170.12.5705-5708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F., Ivey D. M., Ljungdahl L. G. Macromolecular organization of F1-ATPase isolated from Clostridium thermoaceticum as revealed by electron microscopy. J Bacteriol. 1986 Jun;166(3):1128–1130. doi: 10.1128/jb.166.3.1128-1130.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V., Blaut M., Gottschalk G. Generation of a transmembrane gradient of Na+ in Methanosarcina barkeri. Eur J Biochem. 1987 Jan 15;162(2):461–466. doi: 10.1111/j.1432-1033.1987.tb10624.x. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G. Characterization of ferredoxin, flavodoxin, and rubredoxin from Clostridium formicoaceticum grown in media with high and low iron contents. J Bacteriol. 1984 Jan;157(1):1–6. doi: 10.1128/jb.157.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage M. D., Drake H. L. Adaptation of the acetogen Clostridium thermoautotrophicum to minimal medium. J Bacteriol. 1986 Jan;165(1):315–318. doi: 10.1128/jb.165.1.315-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage M. D., Wu Z. G., Daniel S. L., Lundie L. L., Jr, Drake H. L. Carbon monoxide-dependent chemolithotrophic growth of Clostridium thermoautotrophicum. Appl Environ Microbiol. 1987 Aug;53(8):1902–1906. doi: 10.1128/aem.53.8.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano J. S., Schreurs W. J., Kashket E. R. Membrane H Conductance of Clostridium thermoaceticum and Clostridium acetobutylicum: Evidence for Electrogenic Na/H Antiport in Clostridium thermoaceticum. Appl Environ Microbiol. 1987 Apr;53(4):782–786. doi: 10.1128/aem.53.4.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. C., Daniel S. L., Hsu T. D., Drake H. L. Nickel transport by the thermophilic acetogen Acetogenium kivui. Appl Environ Microbiol. 1989 May;55(5):1078–1081. doi: 10.1128/aem.55.5.1078-1081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Dervartanian D. V., Watt G. D. Isolation and characterization of two rubredoxins from Clostridium thermoaceticum. Biochim Biophys Acta. 1980 Mar 7;590(1):24–33. doi: 10.1016/0005-2728(80)90143-7. [DOI] [PubMed] [Google Scholar]



