Abstract
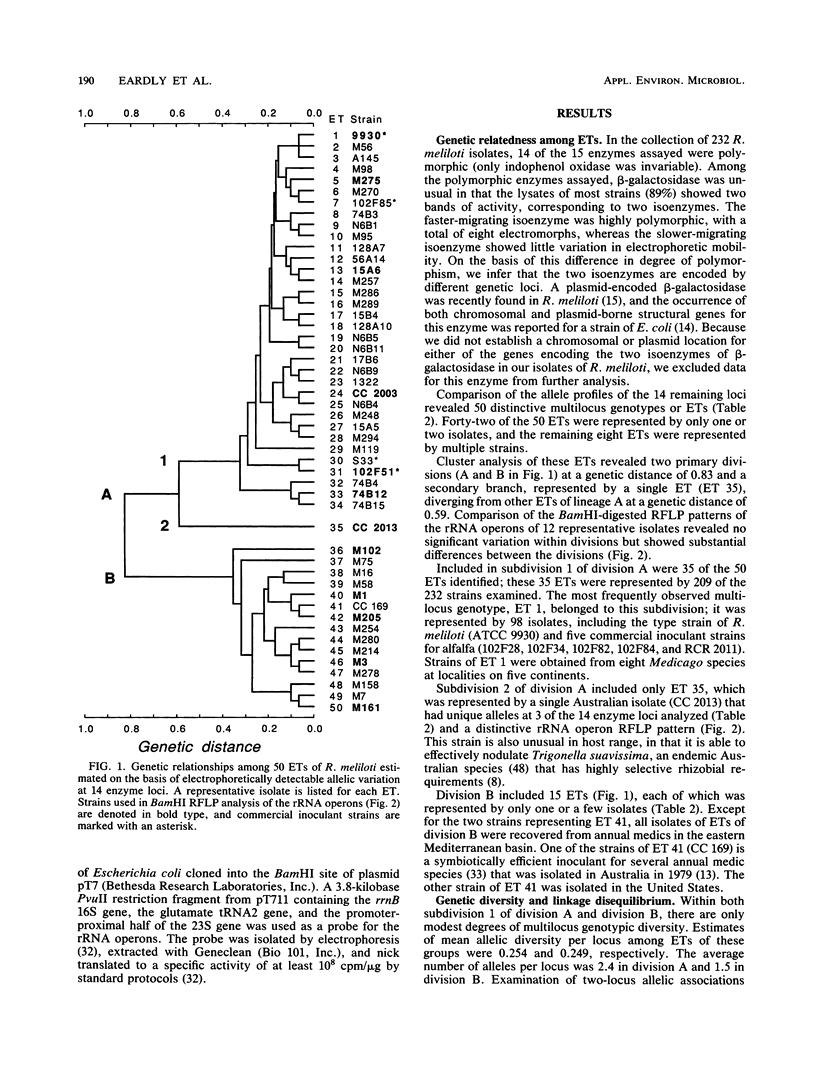
The genetic structure of populations of the symbiotic nitrogen-fixing soil bacterium Rhizobium meliloti was examined by analysis of electrophoretically demonstrable allelic variation in 14 metabolic, presumably chromosomal, enzyme genes. A total of 232 strains were examined, most of which were isolated from southwest Asia, where there is an unsurpassed number of indigenous host species for R. meliloti. The collection consisted of 115 isolates recovered from annual species of Medicago in Syria, Turkey, and Jordan; 85 isolates cultured from two perennial species of Medicago (M. sativa [alfalfa] and M. falcata) in northern Pakistan and Nepal; and 32 isolates collected at various localities in North and South America, Europe, South Africa, New Zealand, and Australia, largely from M. sativa. Fifty distinctive multilocus genotypes (electrophoretic types [ETs]) were identified, and cluster analysis revealed two primary phylogenetic divisions separated at a genetic distance of 0.83. By the criterion of genetic differentiation conventionally applied in defining species limits among members of the family Enterobacteriaceae and certain other bacteria, the two primary divisions of R. meliloti represent distinct evolutionary species. Division A included 35 ETs represented by 209 strains from the eastern Mediterranean basin, northern Pakistan, Nepal, and various other localities worldwide. This division contained the nine commercial alfalfa inoculant strains examined. Division B included 15 ETs represented by 23 isolates, 21 of which were isolated from annual medic species growing in previously uninoculated soils in the eastern Mediterranean basin. The two remaining strains in division B, both representing the same ET, were isolated in the United States and Australia.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfalvi Z., Kondorosi E., Kondorosi A. Rhizobium meliloti carries two megaplasmids. Plasmid. 1985 Mar;13(2):129–138. doi: 10.1016/0147-619x(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Beltran P., Musser J. M., Helmuth R., Farmer J. J., 3rd, Frerichs W. M., Wachsmuth I. K., Ferris K., McWhorter A. C., Wells J. G., Cravioto A. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7753–7757. doi: 10.1073/pnas.85.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny T. P., Gilmour M. N., Selander R. K. Genetic diversity and relationships of two pathovars of Pseudomonas syringae. J Gen Microbiol. 1988 Jul;134(7):1949–1960. doi: 10.1099/00221287-134-7-1949. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Whittam T. S., Kilian M., Selander R. K. Genetic relationships among the oral streptococci. J Bacteriol. 1987 Nov;169(11):5247–5257. doi: 10.1128/jb.169.11.5247-5257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hedrick P. W., Thomson G. A two-locus neutrality test: applications to humans, E. coli and lodgepole pine. Genetics. 1986 Jan;112(1):135–156. doi: 10.1093/genetics/112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanin L., De Lajudie P., Bazetoux S., Huguet T. DNA sequence homology in Rhizobium meliloti plasmids. Mol Gen Genet. 1981;182(2):189–195. doi: 10.1007/BF00269657. [DOI] [PubMed] [Google Scholar]
- Kowalski M. Transducing phages of Rhizobium meliloti. Acta Microbiol Pol A. 1970;2(3):109–113. [PubMed] [Google Scholar]
- Latreille J., Barlogie B., Dosik G., Johnston D. A., Drewinko B., Alexanian R. Cellular DNA content as a marker of human multiple myeloma. Blood. 1980 Mar;55(3):403–408. [PubMed] [Google Scholar]
- McArthur J. V., Kovacic D. A., Smith M. H. Genetic diversity in natural populations of a soil bacterium across a landscape gradient. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9621–9624. doi: 10.1073/pnas.85.24.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero D., Martinez E., Selander R. K. Genetic diversity and relationships among isolates of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1988 Nov;54(11):2825–2832. doi: 10.1128/aem.54.11.2825-2832.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]