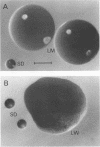
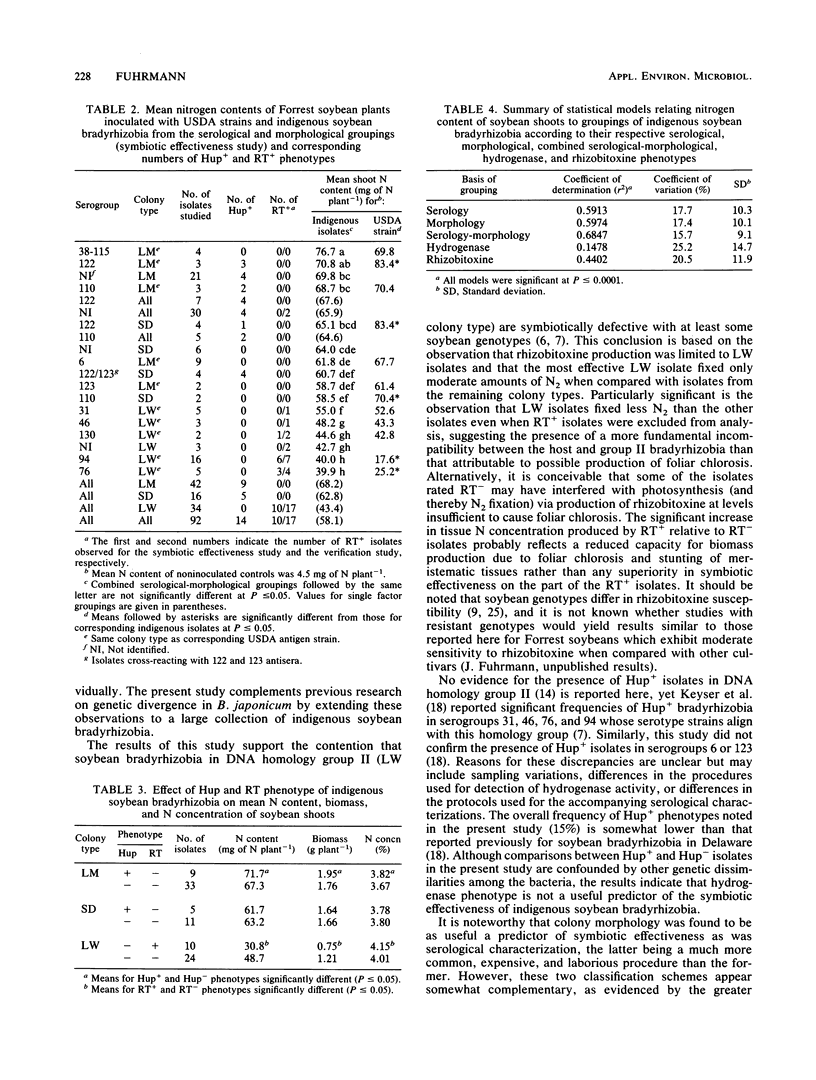
Abstract
A Collection of 360 isolates of Bradyrhizobium japonicum was developed from soybean (Glycine max [L.] Merrill) nodules taken from 18 locations in Delaware. The isolates were characterized serologically with an enzyme-linked immunosorbent assay, morphologically by colony type on yeast extract-mannitol agar, and for production of rhizobitoxine symptoms with soybean plants. These analyses revealed 12 and 3 groups based on serology and morphology, respectively. The more common identifiable isolates were in serogroups 94, 6, 122, and 76. Nearly 33% of the isolates were rated nonreactive with all of the antisera tested. Overall, 18% of the isolates produced rhizobitoxine symptoms, and these were associated with five known serogroups (31, 46, 76, 94, and 130) and the nonreactive grouping, but with only one colony type. A subsample of 92 isolates was rated for N2-fixing ability in the greenhouse and for hydrogenase phenotype in the laboratory. The nitrogen content of plant shoots was strongly and comparably related to both the serological and morphological groupings. Rhizobitoxine and hydrogenase phenotypes were relatively poor predictors of symbiotic effectiveness. Among the serologically reactive isolates, those in serogroups 38-115, 122, and 110 fixed the most N2, whereas one colony type (that containing isolates producing rhizobitoxine) was clearly inferior to the remaining two morphological groupings. Isolates displaying hydrogenase activity (approximately 15% of the isolates tested) correlated with three serologically reactive groupings (serogroups 110 and 122 and a 122/123 cross-reactive group) and two colony types, none of which coincided with groupings containing bradyrhizobia rated positive for rhizobitoxine production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A. K., Keister D. L. Physiology of Ex Planta Nitrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 May;45(5):1592–1601. doi: 10.1128/aem.45.5.1592-1601.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon J. J., Kalia V. C., Heckmann M. O., Salsac L. Influence of the Bradyrhizobium japonicum Hydrogenase on the Growth of Glycine and Vigna Species. Appl Environ Microbiol. 1987 Mar;53(3):610–612. doi: 10.1128/aem.53.3.610-612.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. A., Agarwal A. K., Keister D. L. Extracellular polysaccharide composition, ex planta nitrogenase activity, and DNA homology in Rhizobium japonicum. J Bacteriol. 1984 Jun;158(3):1168–1171. doi: 10.1128/jb.158.3.1168-1171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Weber D. F., Uratsu S. L. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 1984 Apr;47(4):613–615. doi: 10.1128/aem.47.4.613-615.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Rhizobial-Induced Chlorosis in Soybeans: Isolation, Production in Nodules, and Varietal Specificity of the Toxin. Plant Physiol. 1965 Sep;40(5):927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Brown G. G., Verma D. P. Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol. 1985 Jul;163(1):148–154. doi: 10.1128/jb.163.1.148-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P. Expression of uptake hydrogenase and hydrogen oxidation during heterotrophic growth of Bradyrhizobium japonicum. J Bacteriol. 1987 Oct;169(10):4565–4569. doi: 10.1128/jb.169.10.4565-4569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]