Abstract
Background
Direct-to-consumer advertising (DTCA) of prescription drugs has increased rapidly in the United States during the last decade, yet little is known about its effects on prescribing decisions in primary care. We compared prescribing decisions in a US setting with legal DTCA and a Canadian setting where DTCA of prescription drugs is illegal, but some cross-border exposure occurs.
Methods
We recruited primary care physicians working in Sacramento, California, and Vancouver, British Columbia, and their group practice partners to participate in the study. On pre- selected days, patients aged 18 years or more completed a questionnaire before seeing their physician. We asked these patients' physicians to complete a brief questionnaire immediately following the selected patient visit. By pairing individual patient and physician responses, we determined how many patients had been exposed to some form of DTCA, the frequency of patients' requests for prescriptions for advertised medicines and the frequency of prescriptions that were stimulated by the patients' requests. We measured physicians' confidence in treatment choice for each new prescription by asking them whether they would prescribe this drug to a patient with the same condition.
Results
Seventy-eight physicians (Sacramento n = 38, Vancouver n = 40) and 1431 adult patients (Sacramento n = 683, Vancouver n = 748), or 61% of patients who consulted participating physicians on pre-set days, participated in the survey. Exposure to DTCA was higher in Sacramento, although 87.4% of Vancouver patients had seen prescription drug advertisements. Of the Sacramento patients, 7.2% requested advertised drugs as opposed to 3.3% in Vancouver (odds ratio [OR] 2.2, 95% confidence interval [CI] 1.2–4.1). Patients with higher self- reported exposure to advertising, conditions that were potentially treatable by advertised drugs, and/or greater reliance on advertising requested more advertised medicines. Physicians fulfilled most requests for DTCA drugs (for 72% of patients in Vancouver and 78% in Sacramento); this difference was not statistically significant. Patients who requested DTCA drugs were much more likely to receive 1 or more new prescriptions (for requested drugs or alternatives) than those who did not request DTCA drugs (OR 16.9, 95% CI 7.5–38.2). Physicians judged 50.0% of new prescriptions for requested DTCA drugs to be only “possible” or “unlikely” choices for other similar patients, as compared with 12.4% of new prescriptions not requested by patients (p < 0.001).
Interpretation
Our results suggest that more advertising leads to more requests for advertised medicines, and more prescriptions. If DTCA opens a conversation between patients and physicians, that conversation is highly likely to end with a prescription, often despite physician ambivalence about treatment choice.
From 1996 to 2000, spending on direct-to-consumer advertising (DTCA) of prescription drugs in the United States more than tripled,1 reaching US$2.7 billion in 2001.2 The United States and New Zealand are the only industrialized countries that allow such advertising, although restrictive legislation in the European Union3 and Canada4 has recently been under review. Canada allows advertising of over-the-counter (OTC) drugs but prohibits DTCA of prescription medicines, although a 1978 exemption, which was intended to allow price comparisons, permits advertising of product name, price and quantity.4 Nevertheless, Canadians see advertisements in US magazines and on US cable television, as well as an increasing volume of domestically generated DTCA of questionable legality.5 Proponents of DTCA argue that advertisements empower patients, whereas critics counter that they encourage wasteful prescribing.6 Empirical research is needed to assess the effects of DTCA on prescribing decisions, the patient–physician relationship and, ultimately, health outcomes.
We surveyed primary care patients and their physicians in Sacramento, California, and Vancouver, British Columbia. This design allowed us to distinguish between prescriptions requested by patients and those initiated solely by physicians. We hypothesized that US primary care patients would be exposed to more advertising, would request more advertised medicines from their physicians and would receive more prescriptions in response to their requests than similar Canadian patients. Building on earlier work reporting key results from the combined sample,7 we examine how DTCA is affecting prescribing decisions in 2 different policy environments.
Methods
We undertook a comparative cross-sectional survey in primary care physicians' offices in Vancouver from June to August 2000 and in Sacramento from March to June 2001. Two hundred Vancouver physicians were randomly selected from the 2 following lists: clinical faculty with the University of British Columbia's Department of Family Practice (n = 317) and the 1999–2000 Medical Directory of the College of Physicians and Surgeons of British Columbia Vancouver listings for general practitioners (n = 1084). Of these 200 physicians, 155 (78%) were contacted by telephone and 103 met the inclusion criteria. Participants' practice partners were also invited in order to boost participation rates. In Sacramento, we invited 62 primary care physicians who work within the University of California, Davis primary care network. Locums, physicians who provide specialized care to patients referred by their own family physicians and those unavailable during the study period were excluded in both settings.
Patients were included if they were aged 18 years or more, could speak and read English, and could provide informed consent. Patients with severe mental disabilities or psychiatric illness or who looked too ill to complete a questionnaire were excluded.
On pre-selected days, a research assistant invited consecutive patients to participate while in the waiting room, obtained informed consent and gave the patients a questionnaire to complete. Physicians completed a brief questionnaire immediately following consultations with participating patients. The unit of analysis was a paired set of patient–physician questionnaires covering a single consultation.
We measured exposure by asking patients how many different prescription drugs they had seen advertised during the previous year (print and broadcast advertisements) and whether or not they had seen advertisements for 7 listed brands. The patient questionnaire also included self-reported health status (single-item global question), use of health care services (frequency of physician visits and number of OTC and prescription drugs used within the previous 2 weeks), expectations of the consultation, sources of health information, beliefs about patient–physician relationships and medicines, age, sex, household income and drug insurance coverage (full or partial coverage by a third-party payer or full payment out of pocket). The patient questionnaire was designed to focus generally on sources of information about medicines rather than DTCA per se, in order to avoid drawing patients' attention to advertising just before the consultation.
Immediately after the visit, physicians completed a questionnaire listing newly prescribed drugs (≤ 3 per patient) and stating whether or not the patient had “raised the possibility” of using each drug or had directly requested it, or both. We limited questions to up to 3 new prescriptions, because the number of prescriptions arising from most primary care consultations falls within this range.8 We asked whether the physicians would prescribe the same drug to other similar patients using a 3-point scale (“very likely,” “possibly” or “unlikely”) and whether patients were knowledgeable about each drug (yes/no answer). Finally, physicians listed the drugs the patient had requested that had not been prescribed.
We classified a drug as having been advertised to the public if it was among the 50 products with the highest DTCA budgets in the United States in 1999,2 if it featured on a list of television, radio and print advertisements in 2000 and early 2001 obtained from a US market research company9 or if it was listed in a Canadian marketing magazine article on 1999–2000 DTCA campaigns.10
Ethics approval was obtained from the University of British Columbia and the University of California, Davis.
The primary outcome measure was patients' requests for 1 or more prescriptions during surveyed consultations. The required sample size for each study arm was estimated to be 636 paired patient and physician questionnaires, with a hypothesized request rate for DTCA drugs of 6% in the United States and 2% in Canada, and a 50% increase in sample size to allow for the decreased precision expected with cluster sampling. The US rate was based on consumer surveys.11,12 No data were available for Canada. This sample size had 80% power to detect the estimated difference in requests, where α = 0.05 (2-sided).
We examined differences between the 2 samples in self- reported advertising exposure, rates of drug requests and prescribing rates, after adjusting for age, sex, self-reported health status, income and education, and whether patients paid for drugs fully, partially or not at all. Questions about prescribing were also adjusted for physicians' sex and number of years since graduation. We used a generalized estimation equation (GEE) to adjust for correlation between patients of the same physician. This model is an extension of generalized linear modelling that can be used for cluster samples. The outcome is binary, as in logistic regression, but GEE also allows for observations within the same cluster to exhibit some degree of correlation. The GEE analysis was performed using S-PLUS 3.0.13 For potential confounders, regression analysis on correlated variables was used to impute missing values (e.g., education, sex, country and health for missing income data).
Results
Twenty-three of the 103 Vancouver physicians who met the inclusion criteria (22%) agreed to participate, as did 17 of their practice partners. In Sacramento, 38 of 62 physicians who were contacted agreed to participate (61%). The main reason provided for nonparticipation was workload.
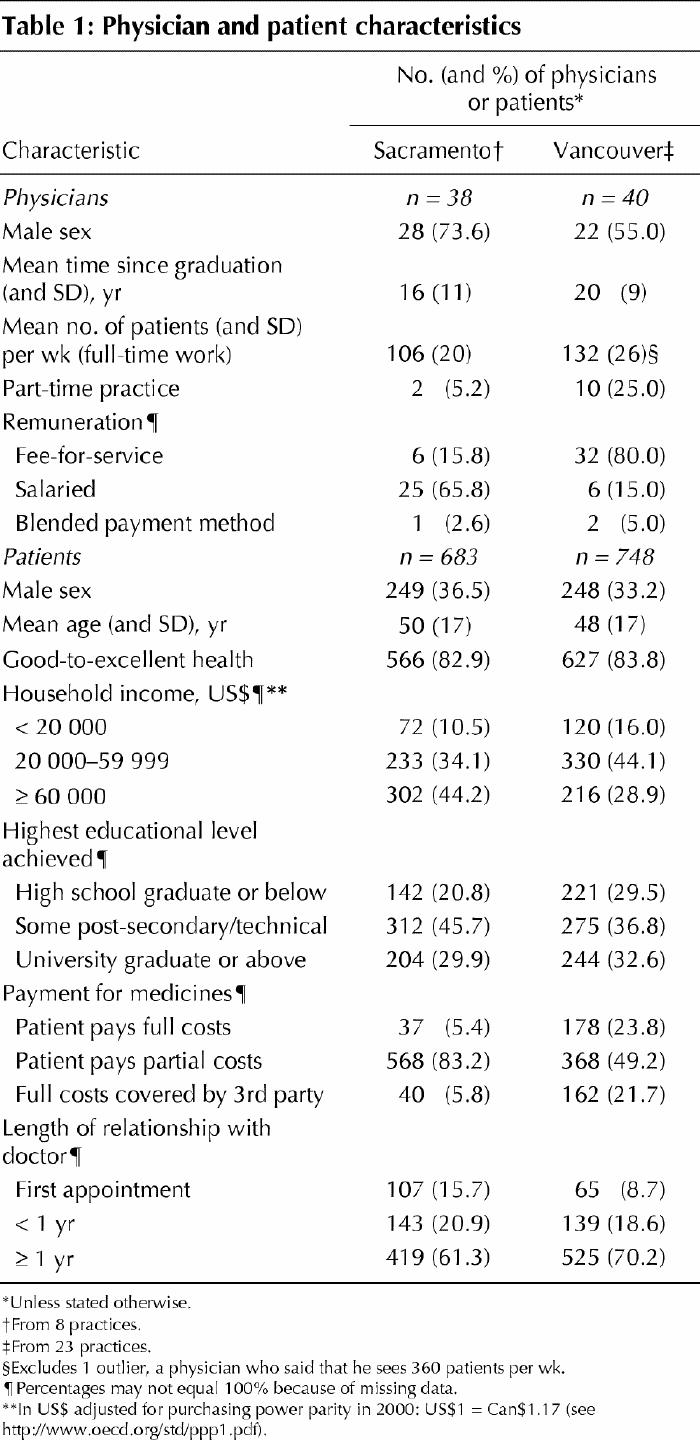
In total, 78 physicians participated in the study: 40 in Vancouver (all family physicians) and 38 in Sacramento (14 general internists and 24 family physicians). The characteristics of participating patients and physicians are described in Table 1. More Sacramento physicians were male (74% v. 55% in Vancouver) and methods of remuneration differed, with most Vancouver physicians being paid on a fee-for-service basis and most Sacramento physicians being on salary. The number of years since graduation and the ratio of men to women did not differ significantly between participating and nonparticipating physicians in either city. Full-time Vancouver physicians saw more patients per week on average than Sacramento physicians, but more Vancouver physicians worked part time.
Table 1
About 61% of consulting patients participated in the survey (n = 1431: 683 in Sacramento, 748 in Vancouver) (Fig. 1). The samples were similar in self-reported health status and demographics; in both cities participant income was higher than average and more patients than expected were of European descent.14,15 More patients in Sacramento than in Vancouver reported partial prescription drug coverage by a third party; more Vancouver patients reported full or no prescription drug coverage.
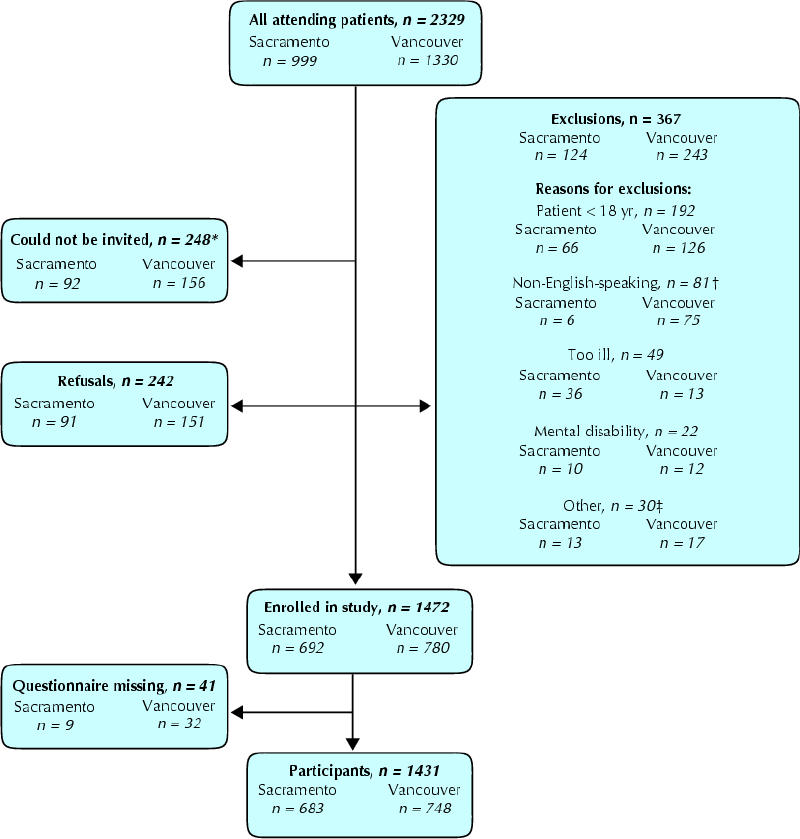
Fig. 1: Patient participation in the study. *These were patients who were directed immediately to the examination room. †The difference was the result of the larger number of non-English speakers and children in Vancouver. ‡Other consists of patients who were not in their physician's office for a consultation (refills, picking up letters), previous participants, patients who were illiterate, deaf or blind, and unspecified (4).
A similar proportion of patients failed to provide income information in the 2 samples (11%), and 7.6% of US patients did not report insurance coverage. Other data on patient characteristics were missing less than 5% of the time.
Attitudes towards the physician–patient relationship and medicines were similar: 75% of patients in each city believed that physicians and patients should have an equal say in treatment, and 14% of patients would go to another physician if their physician refused to prescribe a medicine they wanted. Over 80% of patients mentioned physicians, pharmacists or reference books as their preferred information source on health and medicines; around 1% in each setting listed advertising (data not shown).
Exposure to advertising
Patients in Sacramento were significantly more likely to have seen advertisements for over half of 7 listed products mentioned in the questionnaire and for all individual products except loratadine (Claritin), which is an OTC drug in Canada (Table 2). However, 87.4% of Vancouver patients had seen at least 1 DTC advertisement within the last year and 30% had seen advertisements for more than 10 products.
Table 2
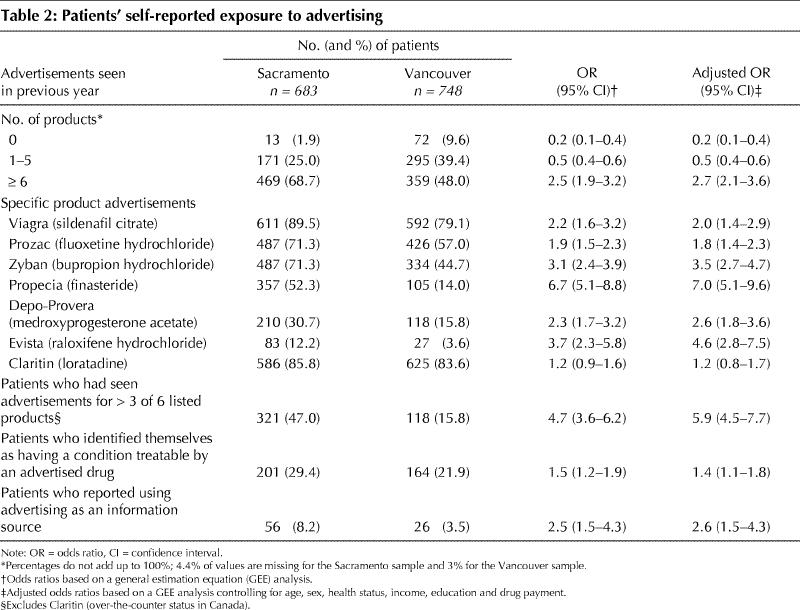
More Sacramento patients (8.2% v. 3.5% in Vancouver) mentioned advertising as contributing to their decision to consult their physician or their belief that they needed a diagnostic test or a medicine, or as an information source they used (adjusted odds ratio [OR] 2.6, 95% confidence interval [CI] 1.5–4.3). More patients in Sacramento also said that they had conditions that could be treated by an advertised drug (29.4% v. 21.9%: adjusted OR 1.4, 95% CI 1.1–1.8). Patients particularly identified their allergies as conditions that could be treated by an advertised drug: 88 (12.9%) in Sacramento compared with 42 (5.6%) in Vancouver.
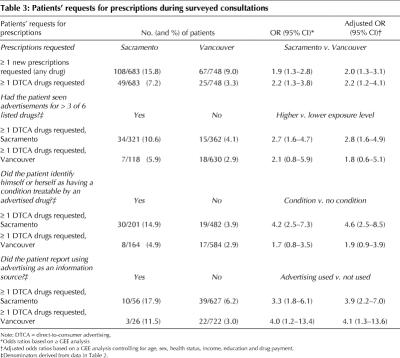
Prescription drug requests
Sacramento patients were twice as likely to request medicines as patients in Vancouver and over twice as likely to request advertised drugs (Table 3). After eliminating 12 consultations in which requested drugs were prescription-only drugs in 1 country and OTC drugs in the other, request rates remained substantially different: 14.2% in Sacramento versus 8.8% in Vancouver (p < 0.01) (data not shown).
Table 3
Advertising exposure was measured through the number of listed products a person had seen advertised, identification with an advertised condition and use of advertising as an information source. In Sacramento, all 3 measures were associated with a higher probability of DTCA drug requests. In Vancouver, only the use of advertising as an information source (3.5% of patients) was significantly associated with DTCA drug requests (Table 3). Fig. 2 compares the number of listed drugs patients had seen advertised with their request rates (χ2 for linear trend = 18.5, p < 0.001).
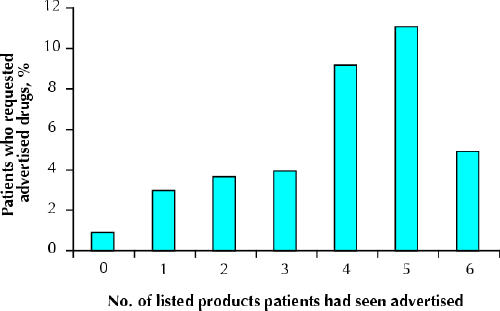
Fig. 2: Proportion of patients who requested DTCA drugs by the number of listed products they remembered having seen advertised. Loratadine (Claritin) was omitted from this analysis, because it had over-the-counter status in Canada. DTCA = direct-to-consumer advertising.
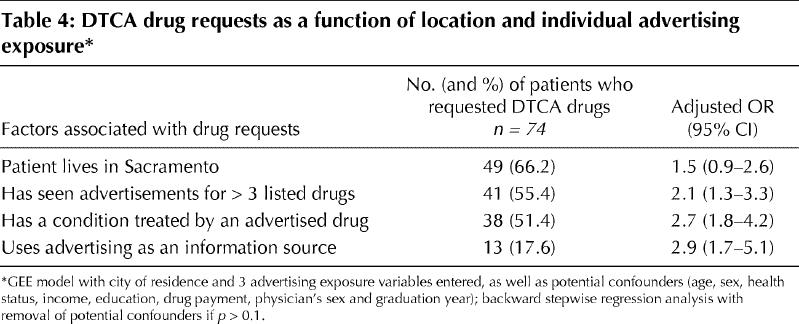
We tested the robustness of city of residence as an independent factor that might influence request rates by including it in the same model as these 3 measures of individual advertising exposure. The coefficient for city of residence became smaller and marginally nonsignificant when adjusted for advertising exposure (OR 1.5, 95% CI 0.9–2.6; p = 0.06); advertising exposure remained highly significant (Table 4).
Table 4
Patients requested 37 different DTCA drugs, 7 of which were requested by ≥ 3 patients. One-quarter of Vancouver DTCA drug requests were for products advertised in Canada.6 The most commonly requested nonadvertised drugs were antibiotics, anxiolytic or hypnotic drugs, and cardiovascular drugs.
Prescribing
More patients in Sacramento than in Vancouver received 1 or more new prescriptions: 41.3% versus 24.9% (adjusted OR 2.1, 95% CI 1.6–2.8; p < 0.01) (Table 5). The prescribing rate was higher overall in Sacramento, but more Vancouver patients received 1 or more refills: 25% versus 18% (data not shown).
Table 5
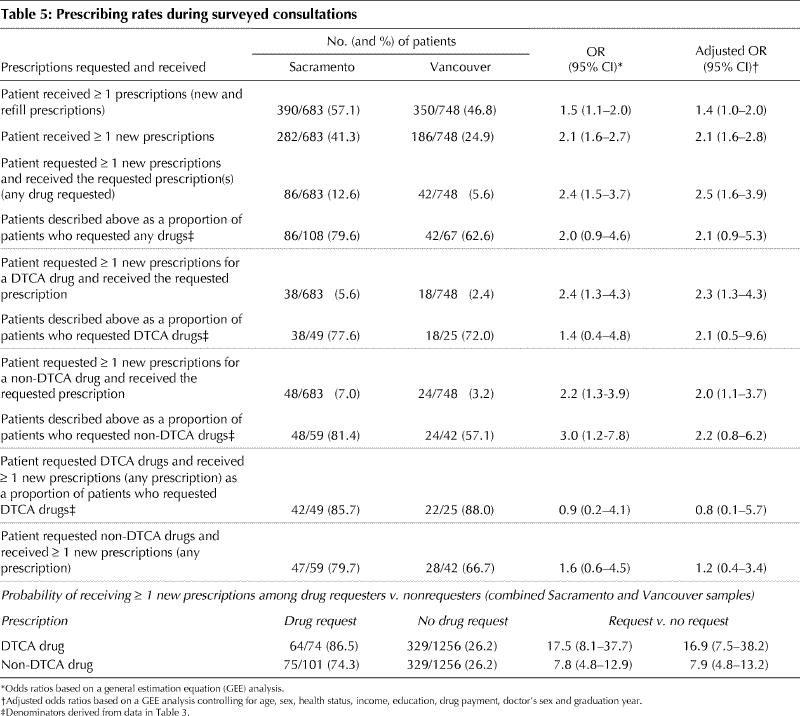
Physicians fulfilled most requests for prescriptions in both settings. In Sacramento 80% of patients who requested prescriptions received them, as compared with 63% in Vancouver (Table 5). The main difference was in the prescribing rate for requested nonadvertised drugs (81.4% v. 57.1%), although this difference was no longer statistically significant after adjusting for patient and physician characteristics (adjusted OR 2.2, 95% CI 0.8–6.2). Prescribing rates for advertised drugs differed less (77.6% v. 72.0%: adjusted OR 2.1, 95% CI 0.5–9.6).
Patients who requested medicines were very likely to receive 1 or more new prescriptions, either for the drugs they requested or alternatives. Indeed, for patients requesting DTCA drugs, the odds of receiving a prescription (for any drug) were 16.9 times those of patients who did not request a medicine (adjusted OR 16.9, 95% CI 7.5–38.2) (Table 5).
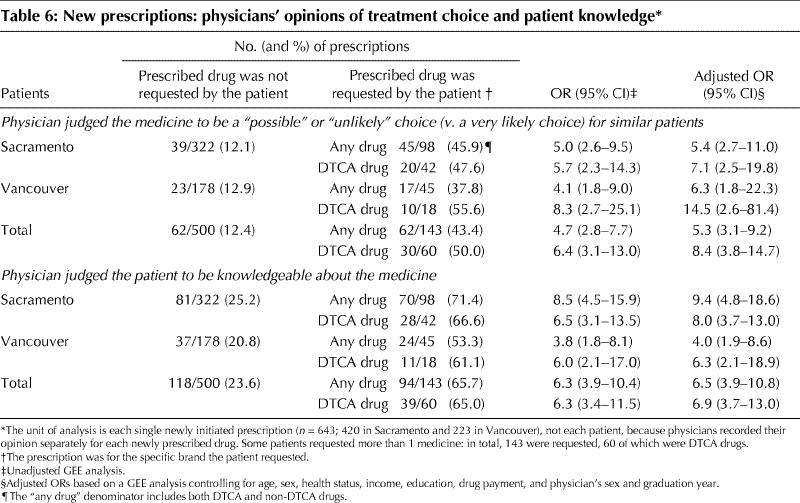
In order to judge physician confidence in treatment choice for each new prescription, we asked, “If you were treating another similar patient with the same condition, would you prescribe this drug?” We judged an answer of “very likely” to indicate confidence in treatment choice, whereas “possibly” or “unlikely” would indicate some degree of ambivalence. In both settings, physicians were more likely to express ambivalence about drugs patients had requested, particularly advertised drugs, than nonrequested drugs (adjusted OR for requested DTCA drugs 7.1 in Sacramento [95% CI 2.5–19.8], 14.5 in Vancouver [95% CI 2.6–81.4]) (Table 6). Physicians were also more likely to judge patients to be knowledgeable about a drug if they had requested it.
Table 6
Interpretation
We found that Sacramento patients reported more advertising exposure and requested more advertised drugs than patients in Vancouver and, in both settings, patients with higher exposure to advertising requested more advertised drugs. The prescribing rate for requested advertised drugs was similar, being about 75%.
Physicians judged 50% of prescriptions for requested DTCA drugs to be a “possible” or “unlikely” choice for similar patients. A key argument made in favour of DTCA is that patients are protected because, ultimately, the physician decides whether or not to prescribe.16 We could not evaluate treatment appropriateness, but if physicians prescribe products that they would not have chosen otherwise, the protection offered by prescription-only status is questionable. In some cases, patients may be right and physicians wrong; however, patients do not obtain sufficient information from advertising to accurately self-diagnose or to choose the best available treatment.17 If physicians are less familiar with a product, they may also be less aware of contraindications, interactions and adverse effects.
DTCA appears to affect prescribing volume as well as product choice. Patients who requested DTCA drugs were nearly 17 times as likely to receive 1 or more new prescriptions as patients who did not request medicines. Nearly 9 of 10 such patients received prescriptions, either for the drug they had requested or an alternative.
Patients' requests for advertised medicines could lead to important health benefits if patients seek and obtain appropriate care, perhaps at an earlier stage, and thus avoid disease complications and admissions to hospital. However, many requested advertised products were “lifestyle drugs”18 or symptomatic treatments. Such therapies may relieve distress or discomfort but are unlikely to prevent admission to hospital or serious morbidity. With a trend toward treatment of milder conditions, a shift may also occur in the balance between expected benefit and potential harm.
We linked self-reported patient exposure to DTCA to patient requests for medicines and prescriptions within surveyed primary care consultations. Other consumer surveys on DTCA have relied on recall of past behaviours over long or indeterminate time periods, introducing a potential for recall bias. We also compared otherwise similar consultations and prescribing decisions that were or were not directly affected by DTCA. This internal comparison group allowed for an examination of the direction of effect of DTCA on prescribing volume and on physicians' confidence in treatment choice. In contrast, the claim made by Weissman and colleagues that DTCA leads to important new diagnoses19 has been criticized because their survey lacked a control group, making it impossible to know whether DTCA leads to fewer or more such diagnoses than occur in other patient visits.20 A physician survey carried out by the US Food and Drug Administration on consultations influenced by DTCA has been subject to similar critique.21
This was a cross-sectional survey based on cluster sampling, and the results are therefore exploratory. Participating physicians may not be broadly representative because of a possible volunteer bias and their links to medical faculties, and the patient population was relatively affluent. In addition, whereas most Sacramento physicians were salaried, Vancouver physicians mainly worked on a fee-for-service basis. This difference is unlikely to explain the higher prescribing rates in Sacramento, because incentives to prescribe are greater under a fee-for-service system.22 The Sacramento survey took place 10 months later than the Vancouver survey, which may have marginally exaggerated observed exposure differences. However, the relation between individual exposure and requests for medicines would not have been affected.
In a comparison of 2 countries, there is a risk of “confounding by culture,” that is, mistakenly attributing cultural differences in behaviour to differences in the advertising environment. However, this cannot account for the finding in both settings that individuals who reported greater advertising exposure had higher request rates for advertised drugs. The most plausible explanation for this consistent relation is an advertising effect. Only the rate at which patients asked for advertised drugs, not physicians' response to requests, differed between the 2 settings.
This survey opens an intriguing window on the effects of DTCA on patient–physician interactions in primary care. Our results are consistent both with a dose-response to advertising at 2 different population exposure levels and, most importantly, with increasing industry investment in this marketing technique.2,23 If DTCA opens a conversation between patients and physicians, that conversation is likely to end with a prescription, despite frequent physician ambivalence about treatment choice. And the greater the patient's exposure to advertising, the more likely such a conversation will occur.
β See related articles pages 421 and 425
Acknowledgments
We thank all of the physicians and patients who participated in the survey.
Footnotes
This article has been peer reviewed.
Contributors: Drs. Mintzes and Barer contributed to study plan, design, analysis and reporting. Drs. Kravitz, Bassett and Lexchin contributed to data interpretation, review of drafts of the manuscript and, to a lesser extent, study plan, questionnaire and data collection. Drs. Kazanjian and Evans contributed to study design, interpretation and review of the manuscript. Dr. Pan contributed to US components of study design and organized and supervised data collection and entry in Sacramento. He also reviewed the manuscript. Dr. Marion contributed to the analysis plan and interpretation and to subsequent discussion of these components and reviewed the manuscript. All authors gave final approval of the version to be published.
This study was funded by Health Canada. Dr. Mintzes also received a PhD fellowship for this research from the National Health Research and Development Programme and the Canadian Institutes of Health Research.
Competing interests: Dr. Pan has received speaker fees from Merck Frosst to produce a public service announcement on varicella immunization. None declared for the remaining authors.
Correspondence to: Dr. Barbara Mintzes, Centre for Health Services and Policy Research, #429–2194 Health Sciences Mall, University of British Columbia, Vancouver BC V6T 1Z3; fax 604 822-5690; bmintzes@chspr.ubc.ca
References
- 1.Rosenthal MB, Berndt ER, Donohue JM, Frank RG, Epstein AM. Promotion of prescription drugs to consumers. N Engl J Med 2002;346(7):498-505. [DOI] [PubMed]
- 2.United States General Accounting Office. FDA oversight of direct-to-consumer advertising has limitations. Report to congressional requesters. Report no GAO-03-177. 2000 Oct. Available: http://rxpolicy.com/studies/gao-dtc-adv.pdf (accessed 2003 July 30).
- 3.Watson R. EC moves towards “direct to consumer” advertising. BMJ 2001;323:184.
- 4.Therapeutic Products Programme. Direct-to-consumer advertising of prescription drugs. Discussion document. Ottawa; Health Canada; 1999.
- 5.Silversides A. Direct-to-consumer prescription drug ads getting bolder. CMAJ 2001;165(4):462. [PMC free article] [PubMed]
- 6.Kravitz RL. Direct-to-consumer advertising of prescription drugs. West J Med 2000:173(4):221-2 [DOI] [PMC free article] [PubMed]
- 7.Mintzes B, Barer ML, Kravitz RL, Kazanjian A, Bassett K, Lexchin J, et al. Influence of direct to consumer pharmaceutical advertising and patients' requests on prescribing decisions: two site cross sectional survey. BMJ 2002;324:278-9. [DOI] [PMC free article] [PubMed]
- 8.Cherry DK, Woodwell DA. National ambulatory care survey: 2000 summary. Advance Data from Vital and Health Statistics no. 328 2002 Jun 5. Available: www.cdc.gov/nchs/data/ad/ad328.pdf (accessed 2003 July 29). [PubMed]
- 9.Video Monitoring Service, Burelle's Information Services. Commercial report: RX 2000 and RX 2001. San Francisco (CA): 2001.
- 10.Young L. Smart pharma tricks. Marketing Magazine 2000;Oct 2:13.
- 11.Consumer and physician attitudes towards direct-to-consumer advertising. New York: Time Inc.; 1998.
- 12.Slaughter E, Schumacher M. Prevention's international survey on wellness and consumer reactions to DTCA of Rx drugs. Emmaus (PA): Prevention Magazine, Rodale Press; 2000/01.
- 13.MathSoft Inc. S-PLUS 2000, version 3.0. 1998-2000; G.E.E. code version 4.13. Available: http://lib.stat.cmu.edu/S/gee (accessed 2003 July 30).
- 14.Sacramento Regional Research Institute. Placer County economic and demographic profile 2003. Sacramento (CA): The Institute; 2003. Available: http://www.placer.ca.gov/business/2003-edp/2003-full-edp.pdf (accessed 2003 July 30).
- 15.1996 Census. Population statistics for Vancouver. Census metropolitan area, British Columbia. Ottawa: Statistics Canada; 1996.
- 16.Holmer AF. Direct-to-consumer prescription drug advertising builds bridges between patients and physicians. JAMA 1999;281(4):380-2 [DOI] [PubMed]
- 17.Bell RA. Wilkes MS, Kravitz RL. The educational value of consumer-targeted prescription drug print advertising. J Fam Pract 2000;49(12):1092-8. [PubMed]
- 18.Lexchin J. Lifestyle drugs: issues for debate. CMAJ 2001;164(10):1449-51. [PMC free article] [PubMed]
- 19.Weissman J, Blumenthal D, Silk AJ, Zapert K, Newman M, Leitman R. Consumers reports on the health effects of direct-to-consumer advertising. Health Affairs 2003:W3(Web exclusive):82-95. Available: www.healthaffairs.org/WebExclusives/Weissman_Web_Excl_022603.htm (accessed 2003 Jul 29). [DOI] [PubMed]
- 20.Bodenheimer T. Two advertisements for TV drug ads. Health Affairs 2003:W3(Web exclusive):112-5. Available: www.healthaffairs.org/WebExclusives/Bodenheimer_Web_Excl_022603.htm. (accessed 2003 Jul 29). [DOI] [PubMed]
- 21.Mitka M. Survey suggesting that prescription drug ads is helping public is met with scepticism. JAMA 2003;289(7):827-8. [DOI] [PubMed]
- 22.Hutchinson JM, Foley RN. Method of physician remuneration and its impact on antibiotic prescription rates. APUA Newsletter 2000;18(3):1-3.
- 23.Morgan S, Mintzes B, Barer M. Direct-to-consumer advertising of prescription only drugs: prescribed to improve consumer welfare? J Health Serv Res Policy. In press. [DOI] [PubMed]