Abstract
ONE OF THE RISK FACTORS FOR HUMAN PAPILLOMAVIRUS (HPV) INFECTION and subsequent lower genital tract neoplasias and cancers is impaired cell-mediated immunity. HIV-positive women with severe immunosuppression are 5 times more likely than HIV-negative women to have lower genital tract neoplasias. A corresponding increase in the risk of invasive vulvar and anal cancers, but not of cervical cancer, has also been observed among HIV-positive women. Treatment failure and recurrence of neoplasia occur much more frequently among HIV-positive than among HIV-negative women. In this review, we discuss recent advances in the understanding of the relation between HIV and HPV coinfection and the development of lower genital tract neoplasias and cancers in women. In addition, we present strategies for monitoring and treating noninvasive and invasive neoplasias of the lower genital tract in HIV-positive women.
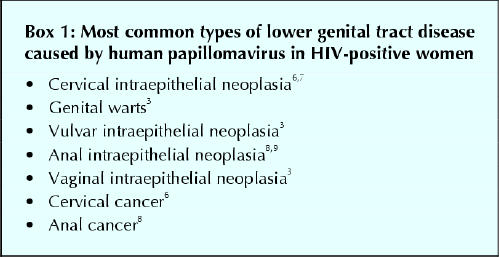
Infection with certain types of human papillomavirus (HPV) has been identified as a cause of cervical intraepithelial neoplasia and cervical cancer and possibly of other neoplasias of the lower genital tract in women.1 One of the risk factors for HPV infection and subsequent neoplasia and cancer of the lower genital tract is impaired cell-mediated immunity.2 Large observational studies involving HIV-positive women have shown a strong and consistent relation between coinfection with HIV and HPV and cervical intraepithelial neoplasia (CIN).3,4,5 It is therefore important for clinicians to screen patients who are HIV-positive women routinely for lower genital tract neoplasias and invasive cancer (Box 1).
Box 1.
In this article, we discuss recent advances in the understanding of the relation between HIV and HPV coinfection and the development of lower genital tract neoplasias and cancers in women. In addition, we present strategies for monitoring and treating noninvasive and invasive neoplasias of the lower genital tract in HIV-positive women.
Cervical intraepithelial neoplasia and HIV infection
HIV-positive women have significantly higher rates of CIN than do HIV-negative women.4,5 In one study, 7% of nearly 400 HIV-positive women had high-grade CIN, as compared with only 1% of 307 HIV-negative control subjects (p < 0.001).4 In another study, CIN was detected by cytological examination in 42% of 273 HIV-positive women and 8% of 161 HIV-negative women; half of the cases found in the HIV-positive group were high-grade lesions.5 Furthermore, it has been shown that HIV infection is a strong risk factor for cervical cancer,1,4 independent of the usual demographic and behavioural risk factors for cervical cancer (Box 2). HIV-positive women with severe immunosuppression (defined as CD4+ cell count below 200 х 106/L) are at greatest risk of CIN.3,4,5 Other risk factors in this cohort of women are age over 34 years, previous treatment of CIN and history of external genital warts.3,4,5
Box 2.

Because HIV-positive women are at increased risk of CIN, one expects that they would have a higher rate of invasive cervical cancer than HIV-negative women would. In 1993, 16 794 cases of women with AIDS were reported to the US Centers for Disease Control and Prevention (CDC); cervical cancer was the most common type of cancer diagnosed in the group (detected in 217 [1.3%]). As a result, the CDC added invasive cervical cancer to its list of AIDS-defining illnesses.6 However, recent studies failed to confirm the high incidence of invasive cervical cancer among HIV-positive women.4,7,11,12 Nevertheless, according to the Sentinel Hospital Surveillance System for HIV infection, the prevalence of cervical cancer among HIV-positive women was almost twice that among HIV-negative women (10.4 per 1000 women v. 6.2 per 1000 women; relative risk [RR] 1.7, 95% confidence interval [CI] 1.1–2.5).11 No increase in invasive cervical cancer has been noted among HIV-positive women in Africa, where both HIV infection and cervical cancer are endemic.12 The reasons for this epidemiological discrepancy are unclear; however, they may be related in part to the rather short lifespan of untreated HIV-positive women in comparison to the 10 years on average required for CIN to progress to invasive cervical cancer.1,7
The effect of highly active antiretroviral therapy (HAART) on the development of CIN in HIV-positive patients has not yet been established. According to the Women's Interagency HIV Study report, women who were taking HAART had higher rates of regression than of progression of CIN, even after adjustment for HPV infection, CD4+ cell count and Papanicolaou (Pap) smear status.13 Others failed to find beneficial effects of HAART on the incidence of CIN detected cytologically.10,14 It is possible that, with long-term experience and histological ascertainment, HAART will be proven to reduce the risk of CIN among HIV-positive women. This is plausible because HAART reverses immunodeficiency, as measured by CD4+ cell counts and interleukin-8 and interleukin-12 levels, and reduces the HIV viral load.15 Long-term longitudinal studies currently underway will help to shed light on the precise influence of HAART on the incidence of invasive cervical cancer in HIV-positive women.
Vulvar and anal neoplasias and HIV infection
As with CIN, lower genital tract condylomata acuminata and vulvar and anal intraepithelial neoplasias are much more common among HIV-positive women than among HIV-negative women.3,8 The prevalence of vulvar intraepithelial neoplasia among HIV-positive women referred for colposcopy as a result of abnormal results of cytological examination varies from 9% to 37%.16 These rates may reflect selection bias: of 396 HIV-positive women who did not attend colposcopy clinics, the prevalence of vulvar intraepithelial neoplasia was only 0.5%. A recent prospective cohort study found pre-existent lower genital tract condylomata or vulvar and anal intraepithelial neoplasias in 6% of 481 HIV-positive women, as compared with 1% of 437 HIV-negative women (p < 0.001).3 In addition, 9% of 385 HIV-positive women, as compared with only 1% of 341 HIV-negative women, had new lesions in the lower genital tract detected during a median follow-up of 3.2 years (p < 0.001); 1 (2%) of the 478 HIV-positive women in whom incident vulvar and anal intraepithelial neoplasias developed had invasive perianal cancer. The incidence of all lower genital tract lesions (excluding cervical) was 16 times higher among HIV-positive than among HIV-negative women.3 A meta-analysis of studies involving more than 50 000 HIV-positive women in the United States found significantly increased risks for in-situ (RR 4.6, 95% CI 4.3–5.0) and invasive (RR 5.8, 95% CI 3.0–10.2) cancer of the vulva or vagina.17 In 2 prospective, cross-sectional studies, anal intraepithelial neoplasia was detected by cytological examination more frequently among HIV-positive women (14% [n = 109] and 26% [n = 102]) than among HIV-negative women (6% [n = 59] and 7% [n = 96]).9,18 In addition, there was a significant correlation between abnormal anal cytological examination results and depressed CD4+ cell counts.
Overall, the relatively high incidence of vulvar and anal intraepithelial neoplasias parallels that of CIN among HIV-positive women. The clinical implication of these findings is the potential for a relatively high number of invasive vulvar and anal cancers in this population. Fortunately, only a few cases have been reported so far.3 The impact of HAART on the progression and treatment outcomes for vulvar and anal intraepithelial neoplasias remains to be determined by long-term cohort studies.
The HIV–HPV connection
More than 40 different types of HPV infect the lower genital tract. Several types of HPV are found in nearly all cases of CIN and cervical cancer; therefore, it is not surprising that the association between cervical cancer and HIV infection is mirrored by a connection between HPV infection and HIV infection. HPV types considered to be of high oncogenic risk (e.g., types 16 and 18) are found in nearly all cases of CIN and cervical cancer and may cause persistent infections.1 They have been correlated with an increased risk of progression from low-grade to high-grade CIN.1 If left untreated, such cases may progress to invasive cervical cancer. According to the Canadian Women's HIV Study, the crude prevalence of HPV infection among asymptomatic, sexually active HIV-positive women aged 15–44 years without CIN at study entry was 73.6% (95% CI 68.5%–78.5%), as compared with only 52.5% (95% CI 46.6%–58.2%) among HIV-negative women with similar demographic profiles.19 The odds ratio was 3.75 (95% CI 2.39–5.89) after controlling for lifetime numbers of sexual partners and history of abnormal cytological examination results. In addition, it has been shown that HIV-positive women are more likely than HIV-negative women to be infected with HPV types of high oncogenic risk, including types 16 and 18, and to be infected with multiple HPV types.2 The cumulative prevalence of persistent HPV infection (more than 2 positive test results) was higher among HIV-positive than among HIV-negative women (87% v. 52%–73%), and the former group was more likely than the latter group to shed HPV DNA continuously (p ≤0.01).2,19 Furthermore, persistent HPV infection was correlated with CD4+ cell counts: it was more readily detected in women with CD4+ counts below 500 х 106/L than in those with higher counts (44% v. 24%; p < 0.01). In a series of 307 HIV-positive women, high HPV load was associated with a 10-fold increased risk of CIN among women with severe immunosupression (CD4+ count below 200 х 106/L) compared with women who had higher CD4+ counts.20
Detection and management of lower genital tract neoplasias in HIV-positive women
The appropriate approach for screening and treating lower genital tract neoplasias in HIV-positive women is subject to debate. This is because of an incomplete understanding of their natural history in this patient population, especially with the advent of HAART. As has been suggested above, it is unclear whether HIV-positive women are truly at increased risk for invasive cervical cancer, although they clearly have a considerably higher prevalence of CIN than their HIV-negative counterparts. Cervical cytological examination to detect precancerous lesions is the most often used screening method among women, including those with HIV infection.6 Currently, the CDC and the US Public Health Service recommend that women undergo 2 Pap tests, 6 months apart, following the initial diagnosis of HIV infection (Box 3); if the results of both tests are negative, annual Pap tests are considered sufficient for routine screening.21,22 This strategy offers quality-adjusted life-expectancy benefits at a cost comparable to that of other clinical preventive interventions.23
Box 3.

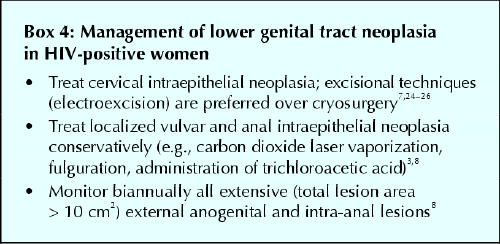
Various strategies have been recommended to manage CIN in HIV-positive women (Box 4). In HIV-negative women, conservative therapies such as cryotherapy for low-grade CIN and loop electrosurgical excision for high-grade CIN are highly successful treatments, with over 80% cure rates.27 However, they are considerably less effective among HIV-positive patients. Rates of treatment failure within 3 years after treatment of CIN in this patient group have ranged from 38% to 62%, as compared with 18% among HIV-negative women.24,25,27 Recurrence rates have been particularly high, reaching 87% in one study involving HIV-positive women who had CD4+ cell counts of less than 200 х 106/L.26 In a pilot study of adjunctive, self-administered maintenance therapy with intravaginal 5-fluorouracil (5-FU) cream after excisional treatment for high-grade CIN in HIV-positive women, the recurrence rate in the treatment group was nearly half that in the group not given the 5-FU cream (28% v. 47%).25 Although the side effects known to occur with intravaginal 5-FU therapy (e.g., vaginal erosions, adenosis and bloody discharge) were not encountered in this pilot study, our experience with this therapy28 suggests that this outcome was unusual.
Box 4.
It is hoped that, with the increasing use of HAART, treatment outcomes for CIN using conservative therapy without adjunctive 5-FU therapy may improve. In one study, HAART was associated with a 35% regression rate in cytologically diagnosed CIN despite persistent HPV infection.14 However, there were only 49 women in the study, follow-up was short (median 5 months), 1 rather than 2 or more cytopathologists read the smears, and the possibility of false-negative cytological test results on follow-up was not ascertained histologically in all cases. Nevertheless, the women who showed signs of regression had higher CD4+ cell counts than those who did not. Because poor response to standard therapies for CIN are related to the CD4+ cell count, restoring immunocompetence in HIV-positive women may improve treatment outcomes for CIN. Indeed, one of the possible reasons for the high rates of treatment failure and recurrence of lower genital tract neoplasias among immunosuppressed individuals, including those with HIV infection, is reactivation of latent HPV infection.29
There are no consensus guidelines for the management of vulvar intraepithelial neoplasia or anal intraepithelial neoplasia in HIV-positive women. Given the relatively high risk of invasive cancer of external anogenital neoplasias in this group of women, a thorough clinical examination of the external anogenital area is mandatory.3 Also, it seems appropriate to use standard ablative or surgical therapies, or both, accompanied by regular long-term follow-up. In a series of 8 cases of untreated vulvar intraepithelial neoplasia in HIV-positive women, 7 progressed to early invasive vulvar cancer.30 Unfortunately, as with CIN, the rates of treatment failure and recurrence among women with vulvar intraepithelial neoplasia and anal intraepithelial neoplasia are high (about 50%–80%), depending on the individual's CD4+ cell count (A.F.: personal observation). So far, monotherapy and combination therapy have failed to demonstrate a decrease in recurrence rates of vulvar and anal intraepithelial neoplasias; the effect of HAART that includes a protease inhibitor has not been evaluated.3 Admittedly, large-scale studies of the natural history of vulvar and anal intraepithelial neoplasias in HIV-positive women using appropriate control subjects are needed to determine the precise rates of treatment failure and recurrence and to relate them to the possible beneficial effect of HAART.
Conclusions
Women who are HIV positive and have HPV infection are at high risk of HPV-related neoplasias of the cervix, vulva and anus. A high risk of invasive cervical cancer has not yet been demonstrated. However, HIV-positive women are at increased risk of invasive vulvar cancer. Gynecological examination should include a thorough inspection of the external anogenital region, and all suspicious lesions should be removed for biopsy. Colposcopy should be performed in response to any abnormal cytological test results, including atypical squamous cells of undetermined significance. Standard treatments of CIN, vulvar intraepithelial neoplasia and anal intraepithelial neoplasia are associated with much higher failure and recurrence rates among HIV-positive women than among HIV-negative women. It remains to be determined whether long-term experience with HAART will result in lower rates of and better therapeutic results for lower genital tract neoplasias in HIV-positive women.
Footnotes
Editor's note: This article was written before new developments emerged regarding the potential for HPV vaccination.
This article has been peer reviewed.
Contributors: Dr. Ferenczy was the principal author. Drs. Coutlée, Franco and Hankins contributed substantially to the acquisition and analysis of data and revised the manuscript critically for important intellectual content. All authors approved the version to be published.
Acknowledgement: We thank Dr. Dan Turner for reviewing an earlier draft of the manuscript.
Competing interests: None declared for Drs. Ferenczy, Coutlée and Hankins. Dr. Franco has received travel assistance from Digene Corporation and GlaxoSmithKline to attend various meetings.
Correspondence to: Dr. Alex Ferenczy, Department of Pathology, Sir Mortimer B. Davis Jewish General Hospital, 3755 Côte Ste-Catherine Rd., Montréal QC H3T 1E2; fax 514 340-7542; alex.ferenczy@mcgill.ca
References
- 1.Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ 2001;164(7): 1017-25. [PMC free article] [PubMed]
- 2.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC Jr. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med 1997;337:1343-9. [DOI] [PubMed]
- 3.Conley LJ, Ellerbrock TV, Bush TJ, Chiasson MA, Sawo D, Wright TC. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet 2002;359:108-13. [DOI] [PubMed]
- 4.Wright TC Jr, Ellerbrock TV, Chiasson MA, Van Devanter N, Sun XW. Cervical intraepithelial neoplasia in women infected with human immunodeficiency virus: prevalence, risk factors, and validity of Papanicolaou smears. New York Cervical Disease Study. Obstet Gynecol 1994;84:591-7. [PubMed]
- 5.Conti M, Agarossi A, Parazzini F, Muggiasca ML, Boschini A, Negri E, et al. HPV, HIV infection, and risk of cervical intraepithelial neoplasia in former intravenous drug abusers. Gynecol Oncol 1993;49:344-8. [DOI] [PubMed]
- 6.AIDS-indicator conditions diagnosed in patients reported in 1993, by age group, United States [Table 12]. HIV/AIDS Surveill Rep 1994;5(4):16. Available: www.cdc.gov/hiv/stats/hivsur54.pdf (accessed 2003 June 23).
- 7.Ellerbrock T, Chiasson M, Bush BA, Sun X, Sawo D, Budney K, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000;283:1031-7. [DOI] [PubMed]
- 8.Palefsky JM. Anal squamous intraepithelial lesions in human immunodeficiency virus-positive men and women. Semin Oncol 2000;27:471-9. [PubMed]
- 9.Williams AB, Darragh TM, Vranizan K, Ochia C, Moss AR, Palefsky JM. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol 1994;83:205-11. [PubMed]
- 10.Hankins C, Money D, Rachlis A, Coutlée F, Wobeser W, Pourreaux K, et al. HAART and evolution of abnormal cervical cytology in women with HIV. XIVth International AIDS Conference; 2002 July 7–12; Barcelona.
- 11.Chin KM, Sidhu JS, Janssen RS, Weber JT. Invasive cervical cancer in human immunodeficiency virus-infected and uninfected hospital patients. Obstet Gynecol 1998;92:83-7. [DOI] [PubMed]
- 12.La Ruche G, Ramon R, Mensah-Ado I, Bergeron C, Diomandé M, Sylla-Koko F, et al. Squamous intraepithelial lesions of the cervix, invasive cervical carcinoma, and immunosuppression induced by human immunodeficiency virus in Africa. Cancer 1998;82:2401-8. [PubMed]
- 13.Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, Melnick S, et al. The effect of highly active antiretroviral therapy on cervical changes associated with oncogenic HPV among HIV-infected women. AIDS 2001;15:2157-64. [DOI] [PubMed]
- 14.Heard I, Schmitz V, Costagliola D, Orth G, Kazatchkine MD. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS 1998;12:1459-64. [DOI] [PubMed]
- 15.Wagner TM, Pezzotti P, Valdarchi C, Rezza G. Different pattern of AIDS-defining diseases in persons responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001;26:394-5. [DOI] [PubMed]
- 16.Korn AP, Abercrombie PD, Foster A. Vulvar intraepithelial neoplasia in women infected with human immunodeficiency virus in Africa. Cancer 1998;82:2401-8. [DOI] [PubMed]
- 17.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000;92:1500-10. [DOI] [PubMed]
- 18.Hillemanns P, Ellerbrock TV, McPhillips S, Dole P, Alperstein S, Johnson D, et al. Prevalence of anal human papillomavirus infection and anal cytologic abnormalities in HIV-seropositive women. AIDS 1996;10:1641-7. [DOI] [PubMed]
- 19.Hankins C, Coutlée F, Girard M, Pourreaux K, Lapointe N, and the Canadian Women's HIV Study Group. Immunosuppression and contraceptive practices associated with persistence of human papillomavirus in HIV-positive and HIV-negative women. XIIIth International AIDS Conference; 2000 July 9–14; Durban, South Africa.
- 20.Heard I, Tassie JM, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among human immunodeficiency virus-infected women with severe immunosuppression and high human papillomavirus load. Obstet Gynecol 2000;96:403-9. [DOI] [PubMed]
- 21.1997 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. USPHS/IDSA Prevention of Opportunistic Infections Working Group. MMWR Recomm Rep 1997; 46(RR-12):1-46. [PubMed]
- 22.1998 guidelines for treatment of sexually transmitted diseases. Centers for Disease Control and Prevention. MMWR Recomm Rep 1998;47(RR-1):1-111. [PubMed]
- 23.Goldie SJ, Weinstein MC, Kuntz KM, Freedberg KA. The costs, clinical benefits, and cost-effectiveness of screening for cervical cancer in HIV-infected women. Ann Intern Med 1999,130:97-107. [DOI] [PubMed]
- 24.Chirenje ZM, Rusakaniko S, Akino V, Munjoma M, Mlingo M. Effect of HIV disease in treatment outcome of cervical squamous intraepithelial lesions among Zimbabwean women. J Low Genital Tract Dis 2003;7:16-21. [DOI] [PubMed]
- 25.Maiman M, Watts DH, Andersen J, Clax P, Merino M, Kendall MA. Vaginal 5-fluorouracil for high-grade cervical dysplasia in human immunodeficiency virus infection: a randomized trial. Obstet Gynecol 1999;94:954-61. [DOI] [PubMed]
- 26.Petry K, Schieffel D, Bode U, Gabrysiak T, Kochel H, Kupsch E, et al. Cellullar immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer 1994;57:836-40. [DOI] [PubMed]
- 27.Spitzer M. Lower genital tract intraepithelial neoplasia in HIV-infected women: guidelines for evaluation and management. Obstet Gynecol Surv 1999;54(2):131-7. [DOI] [PubMed]
- 28.Ferenczy A. Comparison of 5-fluorouracil and CO2 laser for treatment of vaginal condylomata. Obstet Gynecol 1984;64:773-8. [PubMed]
- 29.Khanna N. HAART use in women with HIV and influence on cervical intraepithelial neoplasia: a clinical opinion. J Low Genital Tract Dis 2002;6(2):111-5. [DOI] [PubMed]
- 30.Jones RW. The natural history of vulvar intraepithelial neoplasia. Br J Obstet Gynaecol 1995;102:764-6. [DOI] [PubMed]


