Abstract
To examine the function of prostaglandin (PG) D synthase (PGDS) gene, as well as endogenously produced PGD2 in sleep regulation in vivo, we generated transgenic (TG) mice that overexpress human PGDS gene to study their sleep behavior. Although no difference was observed in the sleep/wake patterns between wild-type and TG mice, a striking time-dependent increase in non-rapid eye movement (NREM), but not in rapid eye movement (REM), sleep was observed in two independent lines of TG mice after stimulation by tail clipping. Concomitantly, the spontaneous locomotor activity of TG animals was drastically decreased in response to the tail clip. Induction of NREM sleep in TG mice was positively correlated with the PGD2 production in the brain. Sleep, locomotion, and PGD2 content were essentially unchanged in wild-type mice after tail clipping. The results with TG mice demonstrate the involvement of the PGDS gene in the regulation of NREM sleep.
Prostaglandin (PG) D2 is the most abundant prostanoid produced in the central nervous system (CNS) of mammals (1) and one of the most potent sleep-inducing substances (2, 3). It has been shown to induce excess sleep in rats (4) and monkeys (5) after intracerebroventricular infusion. Most importantly, sleep induced by PGD2 is indistinguishable from physiological sleep as judged by electroencephalogram (EEG), electromyogram (EMG), brain temperature, locomotor activities, heart rate, and general behavior of animals (4, 5).
PGD2 is produced in the arachidonic acid cascade from a common precursor of various prostanoids, PGH2, by the action of PGD synthase (PGDS) (6). In the CNS, the PGDS was shown to be produced mainly in the leptomeninges and choroid plexus (7) and secreted into the cerebrospinal fluid (CSF) as β-trace (8), the second most abundant protein in CSF after albumin (9). PGD2 is most likely to exert somnogenic activity through PGD2 receptors, dominantly expressed in leptomeninges below the rostral basal forebrain (ref. 10; A. Mizoguchi, Y.U., and O.H., unpublished data). Activation of PGD2 receptors results in subsequent excitation of ventral lateral preoptic neurons, a putative sleep center, which in its turn inhibits the tuberomammillary nucleus, a histaminergic arousal system (11).
PGDS activity in the rat brain and the PGD2 concentration in the CSF of rats reportedly exhibit a circadian fluctuation coupled with sleep/wake cycle, being increased in the light period when rodents mainly sleep (12). Infusion of selective inhibitors of PGDS, e.g., tetravalent selenium compounds, reversibly, time- and dose-dependently inhibited both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep during the daytime (13). These observations clearly showed that PGDS plays a crucial role in the regulation of physiological sleep.
In some sleep disorders such as mastocytosis (14) and African sleeping sickness (15), profound lethargy in patients was proposed to be the result of the overproduction of PGD2. These clinical observations are consistent with the notion that excessive endogenous production of PGD2 induces sleep under certain pathological conditions. However, animal experiments to verify this conclusion have not been done so far. To provide experimental evidence to substantiate this hypothesis, we generated transgenic (TG) mice, in which the human PGDS gene was overexpressed to study the effect of endogenously produced PGD2 on sleep. Here, we report that TG mice exhibit excess of NREM sleep in response to the noxious stimulus, coupled with significant increase in PGD2 production in the brain.
Materials and Methods
Generation of Human PGDS-TG Mice.
A 600-bp fragment containing the coding region of human PGDS cDNA (16) was inserted into the pCAGGS vector under the control of the chicken β-actin promoter and the cytomegalovirus immediate early enhancer (17). This construct then was digested with SalI and NotI and removed from the vector sequence. The resultant 3.1-kb DNA fragment was microinjected into pronuclei of fertilized eggs derived from an inbred FVB/N mouse strain, as described (18). Microinjection was carried out at DNX (Princeton, NJ), and animals then were transferred to the animal facility of the Osaka Bioscience Institute. The founders of the TG mice were identified by Southern blot analysis with the human PGDS gene fragment as a probe. Five independent TG lines were established.
Northern Blot and Enzyme Assay.
Total RNA (10 μg) from different tissues was electrophoresed in an agarose-formaldehyde gel and transferred to the nylon membranes. The RNA was crosslinked to the membrane with UV light at 254 nm (CL-1000 UVP, Upland, CA). The membrane then was hybridized with 32P-labeled probe of the cDNA for human PGDS, exposed on imaging plates, and further analyzed with Fujifilm fluorescent image analyzer FLA 2000. The blot was stripped of the probe and reprobed with cDNA for glyceraldehyde-3-phosphate dehydrogenase.
For detection of PGDS activity, the brains were quickly removed and homogenized in 3 vol of PBS (10 mM sodium phosphate, pH 7.4/150 mM NaCl). The homogenates were centrifuged at 4°C for 15 min at 400,000 × g, and the resulting supernatant was used for PGDS assay in 50 μl of 100 mM Tris⋅HCl (pH 8.0), 40 μM [1-14C]PGH2, and 1 mM DTT (19). The rates of all enzymatic reactions were calculated after subtracting the blank values without the enzyme.
Sleep Monitoring.
Adult specific pathogen-free, male PGDS-TG mice (n = 10–20) and wild-type controls (n = 7–15) of inbred FVB/N strain weighing 25.9 ± 0.9 g (12–15 wk old) were used in the experiments. The animals were maintained under 12-h light/dark cycle (light from 8 a.m. to 8 p.m.) in sound-attenuated temperature-controlled (22.0 ± 1.0°C) chambers. Mice were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and implanted with two EEG electrodes over the parietal cortex (2.0 mm lateral to the midline, 2.5 mm posterior to the bregma) and the cerebellum (1.5 mm posterior to lambda). Two wires were inserted in the muscle of the dorsal neck as EMG electrodes. The electrodes were attached to the skull with dental cement. Animals were allowed at least 10 days of recovery from surgery; and after habituation to experimental conditions, three consecutive 24-h recordings were obtained. After baseline recordings, mice were subjected to tail clipping at the time at which the lights were switched off (8 p.m.) and recorded for the following 2 days. EEG and EMG were recorded with a data acquisition program (Kissei COMTEC, Matsumoto, Japan; NEC Medical Systems, Osaka, Japan), and subsequently analyzed by Animal Sleep program (Kissei COMTEC). The wake, NREM, and REM sleep states were determined for 4-s epochs based on the EEG and EMG recordings according to the criteria as described (20).
Tail clipping performed in the experiments is essentially identical to the routine tail clipping procedure used for the DNA sampling of mice. Tail ends of about 0.5 cm were clipped with scissors without usage of any anesthetic; the mice then were returned to the home cage and further analysis was done.
Locomotor Activity.
Horizontal locomotor activity was detected by 124 infrared photocell beams in an adjustable frame placed over the cage for every individual mouse (Biotex, Kyoto, Japan). After habituation for 1 day before recording, spontaneous locomotor activity was recorded for 48-h intervals, and day-2 recordings served as a baseline. On the third day, tail clipping was performed at 8 p.m., the beginning of the dark period, and the locomotor activity was recorded for another 48-h period.
PGD2 and PGE2 Enzyme Immunoassay.
At specific time points, brains were harvested and immediately frozen in liquid nitrogen. They then were homogenized in ethanol containing 0.02% HCl at pH 2.0 and centrifuged at 500 × g for 20 min. 3H-labeled PGD2 and PGE2 (60 Bq for each per assay) (New England Nuclear) were added as tracers for estimation of the recovery to the supernatant. The PGs were extracted with ethyl acetate, which was evaporated under nitrogen; and the samples then were separated by HPLC (Gilson) (12). The quantification of PGs was performed with a PGD2-MOX enzyme immunoassay kit for PGD2 and a PGE2 enzyme immunoassay kit-monoclonal for PGE2 (Cayman Chemicals, Ann Arbor, MI).
Statistics.
Statistic analysis was performed by two-way ANOVA followed by a post hoc Fisher's probable least-squares difference test to establish the differences between the genotypes and different conditions; analysis of PGs production was performed by an unpaired Student's t test; P < 0.05 was considered to be significant.
Results
For generation of TG mice, we used the cDNA for human PGDS, which was inserted under the control of the chicken β-actin promoter and cytomegalovirus enhancer (Fig. 1A). Northern blot analysis of tissue samples of TG mice showed an excessive amount of mRNA for human PGDS expressed in various organs and tissues of these mice, as expected; whereas mRNA for the endogenous mouse PGDS was seen only in the brain and epididymis, as reported previously (21). Five independent lines of TG mice were generated, and all of the animals appeared to be healthy, grew normally, and produced offspring. Usage of the inbred FVB/N mouse strain for the entire process of transgenesis allowed us to avoid ambiguity caused by different genetic backgrounds (22). When Northern analysis was performed on brain samples from TG mice, the highest expression of mRNA of PGDS was found in B7 and B20 lines (Fig. 1B). Measurement of PGDS activity in the brains of these lines of TG mice revealed that the B7 and B20 mice contained 5- and 2.5-fold greater activity than that of their wild-type counterparts, respectively (Fig. 1C). We used these TG lines to evaluate the role of PGDS in sleep regulation.
Figure 1.
Generation of PGDS-TG mice. (A) Construct of the human PGDS cDNA used for microinjection into the fertilized eggs of FVB/N mouse strain; CMV, cytomegalovirus. (B) Northern blot analysis of total RNA (10 μg/lane) from the brains of five lines of TG mice, using 32P-labeled cDNA probes for human PGDS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (C) PGDS activity in the brains of wild-type and two lines of TG mice (B7 and B20) measured with [14C]PGH2 as the substrate.
All mice exhibited an almost identical circadian pattern in their sleep, and no significant differences were found between wild-type and TG mice (Fig. 2). Analyses of the sleep distribution in the light and dark periods showed that the amounts of NREM (light period: 35.7 ± 1.6, 33.4 ± 3.2, and 34.8 ± 3.2 min/h; dark period: 19.3 ± 2.1, 20.3 ± 1.8, and 18.4 ± 2.3 min/h for wild-type, B7, and B20 TG mice, respectively) and REM (light period: 4.4 ± 0.8, 4.6 ± 0.9, and 4.8 ± 0.8 min/h; dark period: 3.2 ± 0.7, 3.4 ± 0.8, and 3.4 ± 0.9 min/h for wild-type, B7, and B20 TG mice, respectively) sleep were also similar between the genotypes. When spontaneous locomotion of mice was monitored through the dark/light cycle, both wild-type and TG mice displayed a circadian rhythm for locomotor activity, although the basal level of the locomotor activity of TG mice was elevated in comparison with that of the wild-type controls throughout the recording time (Fig. 3). The basal locomotion of B7 mice was about 2-fold higher than that of wild-type mice (Fig. 3 A and B). Locomotor activity of B20 line appeared intermediate between B7 and wild-type mice (Fig. 3C). Thus, the higher locomotor activity of TG mice is attributable to the overexpression of the PGDS gene in almost all tissues, manifesting itself in motor up-regulation.
Figure 2.
NREM and REM sleep in B7 and B20 TG and wild-type mice before and after tail clip. Tail clipping, indicated by arrow, was performed at 8 p.m., the time at which the lights were turned off. Each value is the mean ± SEM estimated from 1 h of baseline recording (○) or after tail clipping (●). (A) NREM and REM sleep were not affected in wild-type mice by the tail clipping. (B) In the B7 mice, tail clipping increased the amount of NREM sleep without affecting the amount of REM sleep. (C) In the B20 line of TG mice, effect of tail clipping was comparable to the B7 mice. n = 7 for wild-type mice, n = 10 for B7, and n = 10 for B20 TG mice; *, P < 0.05; **, P < 0.005; ***, P < 0.0001.
Figure 3.
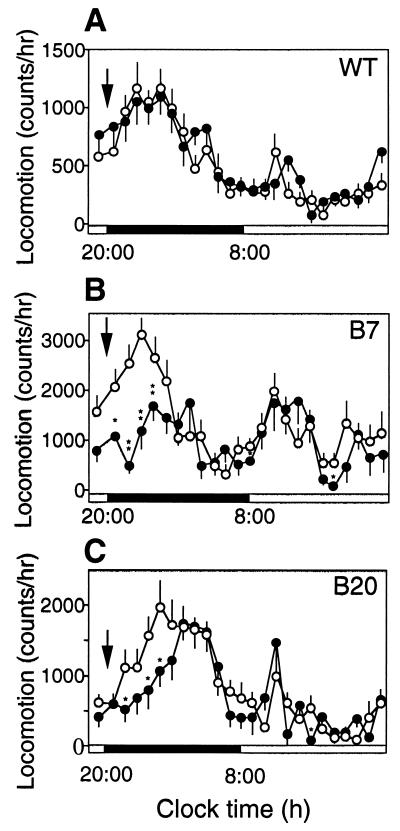
Effect of tail clipping on spontaneous locomotion. (A) Tail clipping, indicated by arrow, applied in the beginning of the dark period (8 p.m.) did not alter locomotor activity of wild-type mice (n = 15), yet decreased the locomotion of B7 (B) (n = 20) and B20 (C) (n = 20) TG mice. Data points are means ± SEM per 1 h of recording. ○, Baseline recordings; ●, after tail clipping. *, P < 0.05; **, P < 0.005.
The major difference between the genotypes was observed after tail clipping, performed for DNA sampling used for the genotyping. TG, but not wild-type, mice appeared sedated by the stimulus. Therefore, we applied tail-clip stimulation to TG and wild-type mice and assessed their sleep by measuring EEG, EMG, and locomotor activity. After tail clipping, the amount of nocturnal NREM sleep of TG mice was significantly higher than that of their wild-type littermate controls (Fig. 2). The effect was apparent in the first hour after stimulation, continued to increase time-dependently, and dissipated within 5 h in both lines of TG mice (Fig. 2 B and C). Slight rebound of wakefulness was noted in the first half of the light period. The maximal 2.5-fold increment of NREM sleep was observed 3 h after the tail had been clipped. At this point, the sleep was almost saturated and comparable to the amount of sleep during the daytime. In contrast, REM sleep was unaffected by the tail clipping in either of the lines of TG mice (Fig. 2 B and C). The sleep pattern of wild-type counterparts after tail clipping was essentially similar to the baseline recordings with only slight decreases in both NREM and REM sleep, which were observed mainly in the dark period (Fig. 2A).
Associated with sleep induction, tail clipping decreased locomotor activity in the TG mice (Fig. 3 B and C). Despite the high basal locomotor activity of these mice, tail clipping done at the beginning of the dark period, when mice are generally the most active, caused a drastic decrease in the locomotor activity of the B7 and B20 mice, in the first 5 h with subsequent recovery, a pattern similar to that seen in the case of sleep induction. Wild-type mice did not change their locomotor activity in response to the tail clipping (Fig. 3A).
To examine whether the induced sleep in TG mice is coupled to the changes in the PGs production, we measured the amounts of PGD2 and PGE2, which are known to induce sleep (2–5) and wakefulness (23), respectively, in the brains of TG and wild-type mice at different time points after their tails had been clipped. The basal amount of PGD2 in TG mice (62.9 ± 5.6 and 75.8 ± 21.1 pg/brain for B7 and B20 mice, respectively) was 1.2- and 1.5-fold greater than that of the wild-type mice (49.8 ± 6.8 pg/brain). The PGD2 amount in the brain of TG mice increased gradually after tail clipping, with a peak representing a 17- and 5-fold increase at 3 h after the clipping (1,051.3 ± 150.9 and 383.9 ± 46.8 pg/brain) of B7 and B20 mice, respectively, and then decreased (Fig. 4). In the case of the wild-type mice, the PGD2 content was slightly increased 1 h after the tail clipping and then returned to the control level (Fig. 4). On the other hand, the basal amount of PGE2 in the brain was comparable between the genotypes for untreated animals (32.0 ± 3.1, 30.4 ± 5.3, and 31.3 ± 3.7 pg/brain for wild-type, B7, and B20 TG mice, respectively). Tail-clip stimulation nonsignificantly increased production of PGE2 in both TG and wild-type mice. However, the extent was much less than that of the PGD2 increase. Thus, the induction of NREM sleep in TG mice is consistent with this increase in the PGD2 production in the brain.
Figure 4.
Time course of PGD2 content in the brain after tail clipping. Tail clip induced production of PGD2 in B7 and B20 TG mice but not in wild-type mice. Time point “0 h” represents the control data, immediately after tail clip. n = 6 for each genotype at each time point; *, P < 0.01; **, P < 0.001 by unpaired Student's t test.
Discussion
This report shows the involvement of the endogenously produced PGD2 in the induction of physiological sleep. Previously, the relationship between PGD2 and sleep was examined mainly pharmacologically by administration of exogenous PGD2 into the brain and inhibition of PGDS in vivo by selenium compounds (3, 4, 10, 13) or under certain pathological conditions as reported in some sleep disorders (14, 15).
Although no differences were found in the baseline recordings of vigilance states between wild-type and TG mice, the tail clipping performed for DNA sampling caused a remarkable increase in nocturnal sleep of TG mice consistent with drastic decrease in locomotor activity, whereas wild-type controls were unaffected by the stimulus (Figs. 2 and 3). Moreover, the sleep induction in TG mice was attributed to the changes in NREM sleep, whereas REM sleep remained undisturbed (Fig. 2 B and C). Despite the high basal locomotor activity of TG mice, tail clipping applied at the beginning of the dark period caused a dramatic decrease in the locomotion of animals in the first 4–5 h (Fig. 3B). This observation is in good agreement with previous reports indicating that i.v. injection of PGD2 produced a significant decrease in the spontaneous locomotor activity in mice and at the same time potentiated pentobarbital-induced sleeping time (24). The tail-clip effect on TG mice was shown to be consistent in two independent lines: B7 and B20 (Figs. 2 and 3). Thus, the increase in the amount of NREM sleep and subsequent decrease in locomotion in TG mice in response to the tail clipping can be interpreted as a result of the overexpression of PGDS and possibly excessive production of endogenous PGD2 rather than the result of the alterations in some candidate genes for sleep regulation caused by the insertion of the transgene.
The interpretation of the current experiments is based on the presumption that the response of TG mice to the tail clipping is because of the initial overexpression of the human PGDS in these mice, and thus increased levels of PGD2. It is well known that when cells are exposed to various exogenous stimuli, such as pain, bacterial lipopolysaccharides, or ethanol, arachidonic acid is released from phospholipids in the cell membrane and is further metabolized by arachidonic acid cascade by the action of cyclooxygenase (COX) to produce PGH2, which is then converted to the individual PGs and thromboxane by tissue-specific synthases, for example, PGDS or PGES (6). The rate-limiting step is generally considered to be the production of PGH2, catalyzed in sequential reaction by phospholipase A2 and COX, rather than the synthase step, under normal conditions. This suggestion is supported by the observation that, in TG mice as well as in wild-type ones, PGDS appeared to be constitutively expressed in the brain and was not induced by tail clipping (data not shown). On the other hand, high amounts of COX-2, an inducible form of COX, were observed in parts of the CNS involved in the processing and regulation of nociceptive, stressful, and inflammatory inputs, and behavioral responses (25, 26). Although the transcripts of COX-2, as well as COX-1, and cytosolic phospholipase A2 were only insignificantly changed in TG and wild-type mice by the stimulation (data not shown), the tail clipping is likely to activate the arachidonic acid cascade at the posttranscriptional level, including enzyme(s) activation, finally resulting in an elevated production of PGH2. The TG mice overexpressed PGDS, which then converted this excess PGH2 into an abundance of PGD2 (Fig. 4). This elevated production of PGD2 induced NREM sleep in clipped TG mice (Fig. 2 B and C). Although the maximal extent of PGD2 production on stimulation differed between B7 and B20 mice, the NREM sleep induction was comparable between these lines (Figs. 2 and 4). This indicates that as little as 5-fold increase in the endogenous PGD2 observed in the brains of B20 mice could induce almost saturable amount of sleep (Figs. 2C and 4). The observation that NREM sleep was induced in TG mice primarily because of the increased production of PGD2 is consistent with the findings that infusion of PGD2 in the rostral basal forebrain of rats effectively and specifically induced NREM sleep (10). Because NREM sleep was selectively affected in these TG mice, it is likely that the PGDS gene is responsible for the regulation of NREM sleep, in contrast to the recently reported orexin/hypocretin gene, which was identified as a gene to be involved in the pathogenesis of narcolepsy and possibly in the regulation of REM sleep (27, 28). Thus, the PGDS-TG mice proved to be a useful animal model to study profound lethargy or excess sleep mediated by PGD2.
Acknowledgments
We thank Profs. I. Tobler, S. Yamamoto, E. Mignot, and M. Yanagisawa for critical reading of the manuscript and valuable comments. We are indebted to Prof. I. Tobler for helpful advice concerning the sleep analyses of mice; we thank Drs. T. Mochizuki, K. Fujimori, Y. Fujitani, and C. Beuckmann for discussions pertaining to the preparation of the manuscript, and Ms. Y. Ono, N. Uodome, and Y. Kuwahata for their technical assistance. This study was supported by grants from the Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency (to Y.U.), the Japan Space Forum (to Y.U.), the Japan Society for the Promotion of Science (Grant 11680642, to E.P.), the Science and Technology Agency (to E.P.), and the Ministry of Health and Welfare of Japan (Grant 100107, to O.H.).
Abbreviations
- PG
prostaglandin
- PGDS
PGD synthase
- EEG
electroencephalogram
- EMG
electromyogram
- REM
rapid eye movement
- NREM
non-REM
- TG
transgenic
- COX
cyclooxygenase
- CNS
central nervous system
- CSF
cerebrospinal fluid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090093997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090093997
References
- 1.Narumiya S, Ogorochi T, Nakao K, Hayaishi O. Life Sci. 1982;31:2093–2103. doi: 10.1016/0024-3205(82)90101-1. [DOI] [PubMed] [Google Scholar]
- 2.Hayaishi O. FASEB J. 1991;11:2575–2581. [PubMed] [Google Scholar]
- 3.Urade Y, Hayaishi O. Biochim Biophys Acta. 1999;1436:606–615. doi: 10.1016/s0005-2760(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 4.Ueno R, Honda K, Inoue S, Hayaishi O. Proc Natl Acad Sci USA. 1983;80:1735–1737. doi: 10.1073/pnas.80.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onoe H, Ueno R, Fujita I, Nishino H, Oomura Y, Hayaishi O. Proc Natl Acad Sci USA. 1988;85:4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urade Y, Hayaishi O. Vitam Horm (San Francisco) 2000;58:90–109. doi: 10.1016/s0083-6729(00)58022-4. [DOI] [PubMed] [Google Scholar]
- 7.Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Proc Natl Acad Sci USA. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann A, Conradt H S, Gross G, Nimtz M, Lottspeich F, Wurster U. J Neurochem. 1993;61:451–456. doi: 10.1111/j.1471-4159.1993.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 9.Clausen J. Proc Soc Exp Biol Med. 1961;107:170–172. doi: 10.3181/00379727-107-26569. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha S S, Kasahara K, Hayaishi O. Proc Natl Acad Sci USA. 1994;91:11998–12020. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scamell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Proc Natl Acad Sci USA. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey H P, Ram A, Matsumura H, Hayaishi O. Biochem Mol Biol Int. 1995;37:431–437. [PubMed] [Google Scholar]
- 13.Matsumura H, Takahata R, Hayaishi O. Proc Natl Acad Sci USA. 1991;88:9046–9050. doi: 10.1073/pnas.88.20.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts J L, II, Sweetman B J, Lewis R A, Austen K F, Oates J A. N Engl J Med. 1980;303:1400–1404. doi: 10.1056/NEJM198012113032405. [DOI] [PubMed] [Google Scholar]
- 15.Pentreath V W, Rees K, Owolabi O A, Philip K A, Doua F. Trans R Soc Trop Med Hyg. 1990;84:795–799. doi: 10.1016/0035-9203(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 16.Nagata A, Suzuki Y, Igarashi M, Eguchi N, Toh H, Urade Y, Hayaishi O. Proc Natl Acad Sci USA. 1991;88:4020–4024. doi: 10.1073/pnas.88.9.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 18.Taketo M, Schroeder A C, Mobraaten L E, Gunning K B, Hanten G, Fox R R, Roderick T H, Stewart C L, Lilly F, Hansen C T, Overbeek P A. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urade Y, Fujimoto N, Ujihara M, Hayaishi O. J Biol Chem. 1987;262:3820–3825. [PubMed] [Google Scholar]
- 20.Tobler I, Deboer T, Fischer M. J Neurosci. 1997;17:1869–1879. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujihara M, Urade Y, Eguchi N, Hayashi H, Ikai K, Hayaishi O. Arch Biochem Biophys. 1988;260:521–531. doi: 10.1016/0003-9861(88)90477-8. [DOI] [PubMed] [Google Scholar]
- 22.Valatx J-L, Douhet P, Bucchini D. J Sleep Res. 1999;8:65–68. doi: 10.1046/j.1365-2869.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura H, Honda K, Goh Y, Ueno R, Sakai T, Inoue S, Hayaishi O. Brain Res. 1989;481:242–249. doi: 10.1016/0006-8993(89)90800-7. [DOI] [PubMed] [Google Scholar]
- 24.Hollingsworth E B, Patric G A. Pharmacol Biochem Behav. 1985;22:365–370. doi: 10.1016/0091-3057(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Breder C D, Dewitt D, Kraig R P. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Banion M K. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qui X, de Jong P J, Nishino S, Mignot E. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 28.Chemelli R M, Willie J T, Sinton C M, Elmquist J K, Scamell T, Lee C, Richardson J A, Williams S C, Xiong Y, Kizanuki Y, et al. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]