Abstract
Familial Danish dementia (FDD), also known as heredopathia ophthalmo-oto-encephalica, is an autosomal dominant disorder characterized by cataracts, deafness, progressive ataxia, and dementia. Neuropathological findings include severe widespread cerebral amyloid angiopathy, hippocampal plaques, and neurofibrillary tangles, similar to Alzheimer's disease. N-terminal sequence analysis of isolated leptomeningeal amyloid fibrils revealed homology to ABri, the peptide originated by a point mutation at the stop codon of gene BRI in familial British dementia. Molecular genetic analysis of the BRI gene in the Danish kindred showed a different defect, namely the presence of a 10-nt duplication (795–796insTTTAATTTGT) between codons 265 and 266, one codon before the normal stop codon 267. The decamer duplication mutation produces a frame-shift in the BRI sequence generating a larger-than-normal precursor protein, of which the amyloid subunit (designated ADan) comprises the last 34 C-terminal amino acids. This de novo-created amyloidogenic peptide, associated with a genetic defect in the Danish kindred, stresses the importance of amyloid formation as a causative factor in neurodegeneration and dementia.
Heredopathia ophthalmo-oto-encephalica or familial Danish dementia (FDD) was originally described by Strömgren and collaborators, who identified nine cases in three generations of a dominantly inherited disorder originating in the Djursland peninsula, northeast of Århus, Denmark (1, 2). The disease is characterized by the progressive development of cataracts and other ocular disorders (including ocular hemorrhages), hearing impairment, varying neurological symptoms, and dementia, usually associated with paranoid reactions and temporal disturbance of consciousness. Cataracts seem to be the first manifestation of the disease, starting before the age of 30, whereas impaired hearing normally develops 10–20 years later. Cerebellar ataxia occurs shortly after the age of 40, followed by paranoid psychosis and dementia 10 years later. Most patients die in their fifth to sixth decade of life. Neuropathologically, the disease is characterized by (i) a uniform diffuse atrophy of all parts of the brain; (ii) a very severe chronic diffuse encephalopathy, mostly in the cerebellum, the cerebral cortex, and the white matter; and (iii) the presence of extremely thin and almost completely demyelinated cranial nerves. A widespread amyloid angiopathy is present in the blood vessels of the cerebrum, choroid plexus, cerebellum, spinal cord, and retina. The presence of plaques and neurofibrillary tangles is the major histopathological finding in the hippocampus, whereas the cerebral white matter also shows some ischemic lesions (2, 3). Herein, we report the isolation and biochemical characterization of a de novo-created amyloid protein and the identification of the genetic defect in the BRI gene that results in dementia in the Danish kindred.
Materials and Methods
Isolation and Biochemical Characterization of Danish Amyloid (ADan).
The deposited amyloid was isolated from leptomeninges of a patient with FDD (case IV1; Fig. 6B). Briefly, the leptomeningeal vessels were dissected and washed three times with 100 mM Tris⋅HCl/2 mM EDTA (pH 7.4), followed by centrifugation at 16,000 × g to eliminate blood contaminants. The vessels were homogenized in 50 mM Tris, pH 7.5, containing 10 mM CaCl2, and digested with a combination of collagenase and DNaseI (Sigma). Amyloid was extracted from the remaining insoluble pellet by incubation with 99% formic acid for 2 h at room temperature. The formic acid-soluble material was dried under a N2 atmosphere, analyzed on a 16% Tris⋅Tricine SDS/PAGE, electrotransferred onto polyvinylidene difluoride membranes (Immobilon-P, Millipore) by using 10 mM 3-cycloexylamino-1-propanesulfonic acid (CAPS) buffer, pH 11, containing 10% methanol, and the corresponding band subjected to N-terminal sequence analysis on a 477A protein sequencer with an on-line 120A PTH analyzer (Applied Biosystems). For MS studies, the formic acid extract was subjected to matrix-assisted laser desorption ionization/MS analysis at the Harvard Microchemistry Facility, Harvard University.
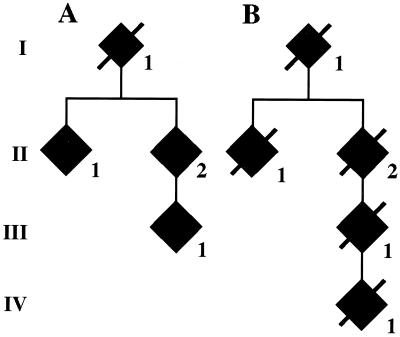
Figure 6.
Analysis of the 10-nt duplication in the BRI precursor protein gene in the Danish kindred. Members of the Danish family pedigree are shown in A and B. Roman numbers indicate different generations. Only affected members (filled symbols) are indicated. Genomic DNA was isolated from blood samples of cases AII1, AII2, and AIII1, and from formalin tissue of samples BII2 and BIII1 and from brain tissue of case IV1. In all cases, the 10-nt duplication was determined after cloning and sequencing of the amplified DNA.
Production of Antibodies.
Polyclonal antibody (Ab 5282) was raised in New Zealand White rabbits by using a synthetic peptide homologous to residues 22–34 (CFNLFLNSQEKHY) of the ADan protein coupled to keyhole limpet hemocyanin through the N-terminal Cys as immunogen (4). After an initial s.c. stimulation with 200 μg of antigen emulsified in RIBI adjuvant, animals were boosted with 50 μg of antigen every 3 wk for 12 wk. The presence of specific antibodies was tested by ELISA and dot blot analysis by using a synthetic peptide homologous to the full-length ADan sequence.
Western Blot Analysis.
Leptomeningeal amyloid proteins were electrophoresed on a 16% Tris⋅Tricine-SDS/PAGE and subsequently transferred for 1 h at 400 mA at 4°C onto Immobilon-P by using CAPS buffer. The membranes were blocked with 5% nonfat dry milk in 10 mM phosphate buffer/137 mM NaCl/2.7 mM KCl (PBS), pH 7.4, containing 0.1% Tween-20 (PBS-T) and subsequently incubated for 2 h at room temperature with polyclonal anti-ADan (Ab 5282; 1:3,000 in PBS-T) and anti-British amyloid (ABri) (4) (Ab 338; 1:3,000 in PBS-T). Horseradish peroxidase-conjugated goat anti-rabbit (Amersham) was used as the secondary antibody (1:5,000 in PBS-T). The immunoblots were visualized by chemiluminescence (Amersham) according to the manufacturer's specifications.
Immunohistochemical Studies.
Sections were deparaffinized in xylene and rehydrated with decreasing concentrations of ethanol. Tissue was pretreated with 99% formic acid for 10 min and endogenous peroxidase activity quenched by incubation with 0.3% H2O2 in methanol for 10 min. After blocking nonspecific staining by incubation with normal swine serum, the tissue sections were reacted with Ab 5282 (1:2,000) for 1 h at room temperature followed by biotinylated anti-rabbit Ig (Dako 1:200) for 30 min and ABC complex (Dako) for another 30 min. Color was developed with diaminobenzinedine/H2O2. Staining for ABri was performed by using Ab 338 as described (4). For β-amyloid protein (Aβ) immunohistochemistry, monoclonal Ab 6F/3D (Dako, 1:100) was used followed by an anti-mouse Ig (Dako, 1:200) as secondary antibody. For τ immunohistochemistry, we used either the polyclonal Ab A024 (Dako, 1:200) or monoclonal Ab AT8 (Innogenetics, Alheretta, GA, 1:50). In both cases, the formic acid pretreatment was omitted, and anti-rabbit (Dako 1:200) or anti-mouse (Dako, 1:200) secondary antibodies were used, as appropriate.
Genetic Analysis.
Amplification of genomic DNA samples isolated from peripheral blood leukocytes of living FDD family members, as well as brain tissue obtained postmortem, was performed as described in ref. 4, by using oligonucleotide primer pairs F: 5′-CGT GAA GCC AGC AAT TGT TTC GCA-3′ and R: 5′-AGCCCTGTTTGCTACTTACATG-3′ (product = 191 bp) by PCR by using 250 μmol dNTPs/2.5 mM MgCl2/50 pmol oligonucleotides, in 100 μl, cycled for 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s. Restriction enzyme analysis of the amplified PCR products was performed with XbaI (GIBCO/BRL) according to the manufacturer's protocol, and the resulting products were resolved by nondenaturing 5% PAGE. PCR amplification by using oligonucleotide primer pairs F and R2: 5′-ACC AAA TTG TAA AGG GTG GG -3′ was done by using 250 μmol dNTPs, 2.5 mM MgCl2, 50 pmol oligonucleotides, in 100 μl, for 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s. PCR products were cloned into pCR 2.1 vector (Invitrogen) and sequenced by automated cycle sequencing in both directions. Amplified DNA fragments were run on a 4% Metaphor gel and the resulting bands visualized by ethidium bromide staining.
Results
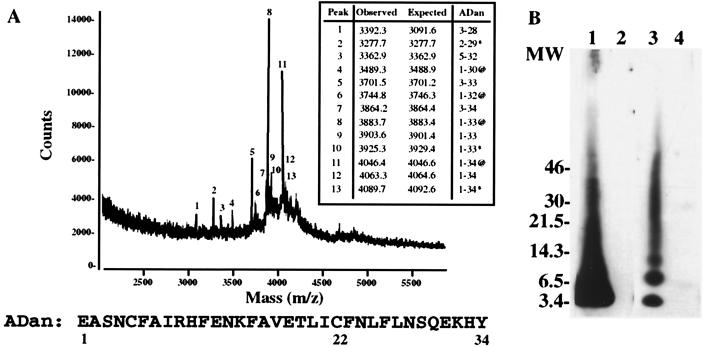
Amino acid sequence analysis of the 4-kDa ADan subunit (Fig. 1), purified from leptomeningeal vessels of an affected Danish patient, yielded the sequence SNXFAIRHFENKFAVE that represented ≈5% of the expected protein yield. blastp homology search (http://www.ncbi.nlm.nih.gov/blast) of this sequence indicated homology with two putative integral transmembrane proteins named E3–16 (AF092128) and E25B (or ITM2B protein) (5, 6). The amino acid sequence also had homology to the recently described ABri peptide starting at position three (4). Restriction enzyme analysis of PCR amplified gene BRI between nucleotides 727 and 918 by using oligonucleotides F and R indicated the absence of the XbaI restriction site in a case with FDD (Fig. 2A, lane 2). The XbaI site is characteristic of patients affected with familial British dementia (FBD) (Fig. 2A, lane 3). When the BRI gene (nucleotides 727–868) was amplified by PCR by using oligonucleotides F and R2, two amplification products of 141 and 151 bp were observed in the Danish case after electrophoresis onto 4% Metaphor agarose gel, whereas normal controls featured only the 141-bp product (Fig. 2B). DNA sequence analysis of the amplified fragments revealed the presence of a 10-nt duplication of nucleotides 786–795 (795–796 insTTTAATTTGT), between codons 265 and 266 of the normal precursor protein BRI cDNA (4) (Fig. 3). The decamer duplication is located just one codon before the normal stop codon (267) and results in a frame-shift of the BRI gene that now extends up to the next in-frame stop codon, generating a precursor protein of 277 amino acids instead of 266 amino acids. Matrix-assisted laser desorption ionization-time-of-flight MS analysis of the purified leptomeningeal ADan indicated the presence of two prominent peaks of 3,883.7 and 4,046.4 mass units (Fig. 4A). The 4,046.4-Da peptide (peak 11) matched exactly the estimated mass of a peptide encoded by the last 34 amino acids of the mutated precursor in Danish patients carrying the insertion (Fig. 3), with the N-terminal amino acid corresponding to a cyclic pyroglutamate. The second major peptide (peak 8) showed a molecular mass of 3,883.7 Da matching the mass of the amyloid molecule extending from residues 1 to 33 with cyclic pyroglutamate at the N terminus and a detyrosinated C terminus. Minor peaks 1, 5, and 7 corresponded to peptides starting at position 3 of the ADan molecule and extending to residues 28, 33, and 34, respectively. The presence of these minor nonblocked peptides accounted for the above-described direct N-terminal sequence results. Western blot studies using polyclonal antibodies raised against a synthetic peptide homologous to amino acid residues 22–34 of the ADan molecule (Ab 5282) specifically immunolabeled the leptomeningeal ADan (Fig. 4B, lane 1) and did not react with leptomeningeal ABri isolated from a case of FBD (Fig. 4B, lane 2). In addition, polyclonal Ab 338, raised against a synthetic peptide homologous to the last 11 residues of the ABri peptide, immunoreacted with ABri (Fig. 4B, lane 3) and did not react with ADan in blots (Fig. 4B, lane 4) or brain lesions in three cases of FDD (not shown). Absorption of the antibodies with the corresponding synthetic peptides used as immunogen abolished the immunoreactivity in all cases.
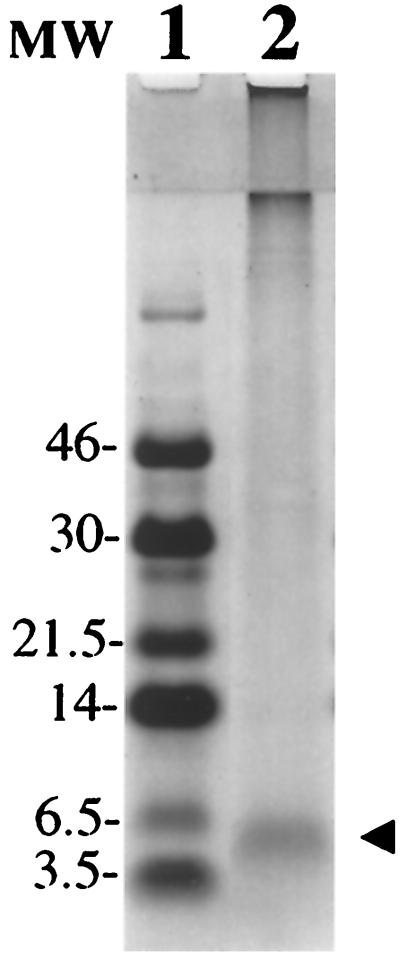
Figure 1.
Isolation of ADan. Tris⋅Tricine SDS/PAGE (16%) of the amyloid protein isolated from leptomeningeal vessels stained with Coomassie blue. The 4-kDa band (line 2, arrowhead) was subjected to Edman N-terminal amino acid sequence analysis after being transferred onto an Immobilon-P membrane. Line 1: Low molecular-mass markers (Amersham) in kDa.
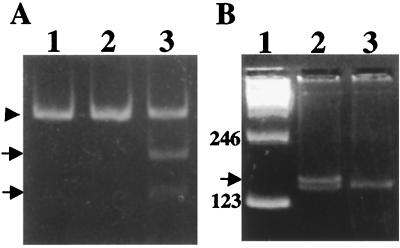
Figure 2.
Analysis of PCR-amplified genomic DNA in the Danish kindred. (A) Polyacrylamide gel showing the restriction enzyme analysis performed as described on ref. 4. Genomic DNA was amplified with oligonucleotides F and R and analyzed with XbaI from a normal control (lane 1), a patient with FDD (lane 2), and a patient with FBD (lane 3). The arrowhead indicates undigested DNA (191 bp), and the arrows indicate digested fragments of 116 and 75 bp. (B) Metaphor agarose gel (4%) of PCR-amplified fragments by using primers F and R2. Lane 1: 123 molecular-mass marker. Lane 2: PCR product of amplification of the BRI gene in a patient with FDD. Lane 3: PCR amplification of a normal control. Amplification in the same conditions by using genomic DNA from patients with FBD gave identical results as seen in normal controls. The extra band observed in the Danish kindred (151 bp) is indicated by an arrow.
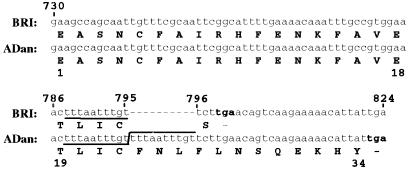
Figure 3.
Nucleotide and amino acid sequence of ADan. Nucleotide and translated amino acid sequence of the wild-type BRI gene between nucleotides 730 to 824 and the corresponding sequence in amyloid ADan. A gap in the wild-type BRI sequence was added to show the insertion of the 10 nucleotides that are duplicated (underlined) in FDD patients. Nucleotides are numbered in the 5′ to 3′ orientation. Termination codons are noted in bold. Amino acid sequences are in one-letter code.
Figure 4.
MS and Western blot analysis of leptomeningeal ADan. (A) MS analysis of the leptomeningeal amyloid isolated from the Danish case. * indicates formylated species (+28 units of mass), and @ indicates species with pyroglutamate at the amino terminus (−18 units of mass). The sequence of the full-length amyloid (34 amino acids) is indicated in one-letter code. (B) Western blotting analysis of purified amyloid fibrils from leptomeningeal vessels of cases with FDD (lanes 1 and 4) and FBD (lanes 2 and 3) reacted against anti-ADan antibody (5282) (lanes 1 and 2) and anti-ABri antibody (338) (lanes 3 and 4). Molecular masses are in kDa.
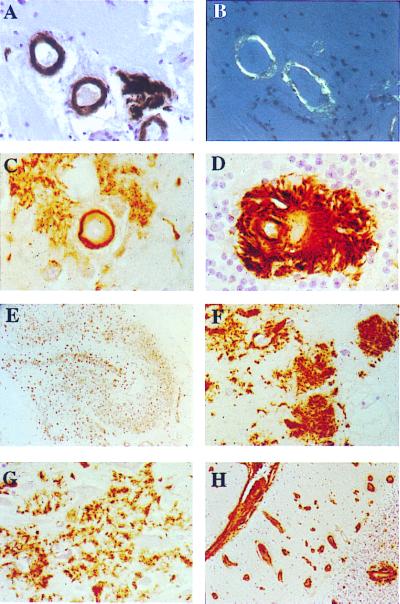
Immunohistochemical studies using the Ab 5282 specifically labeled Congo red positive, parenchymal, and vascular amyloid lesions in addition to Congo red negative deposits in brain tissue of three FDD cases (Fig. 5A–D). These deposits did not react with Ab 338. The hippocampus, subiculum, and enthorhinal cortex showed extensive Ab 5282 immunoreactive deposits, which included several morphological subtypes (Fig. 5E). In the CA4 subregion of the hippocampus, in addition to vascular amyloid, numerous 60- to 120-μm large loosely formed Ab 5282-positive, mostly Congo red negative deposits, some with cotton-wool ball-like appearances or a perivascular localization, were seen (Fig. 5F). The inner molecular layer of the dentate gyrus contained numerous Congo red negative cotton wool-like plaques, whereas the remaining subregions of the hippocampus were densely occupied by loose less well circumscribed Congo red-negative deposits with a tendency to form irregular confluent structures (Fig. 5G). Many plaques in the entorhinal cortex were well formed and compact in appearance, similar to those of the dentate gyrus. The neocortical areas showed characteristic Ab 5282-positive vascular amyloid and perivascular deposits. The cerebellum was one of the most affected tissue structures, with severe leptomeningeal and parenchymal amyloid angiopathy as well as perivascular plaques (Fig. 5 D and H). No staining was observed using preimmune serum or by omitting the primary antibody. Immunohistochemical staining of brain tissue from patients with FBD, hereditary cerebral hemorrhage with amyloidosis Dutch type, and Alzheimer's disease (AD) was negative for Ab 5282. Aβ immunohistochemistry showed variable colocalization of this peptide with Ab 5282-positive vascular amyloid mainly in the form of perivascular deposits. Furthermore, the walls of some blood vessels were positive for Aβ in the hippocampus. τ-Immunoreactive structures were numerous in the hippocampus where abnormal neurites, mostly around affected blood vessels, neurofibrillary tangles, and neuropil threads were seen in large numbers (not shown). Although neocortical areas were less affected by these changes, neurofibrillary tangles, neuropil threads, and an arrangement of abnormal τ-positive neurites were seen around blood vessels with amyloid deposition.
Figure 5.
Immunohistochemical analysis. Parenchymal vessels of temporal sections (A) immunostained with Ab 5282 (×40) and (B) observed under polarized light after Congo red staining (×40). Examples of amyloid angiopathy with perivascular deposits in the subiculum (C) and granular cell layer of the cerebellar cortex (D) (Ab 5282; ×200). All regions of the hippocampus contain numerous Ab 5282-positive plaques (E) giving a good outline of the anatomy of the hippocampus (Ab 5282; ×4). Cotton wool-like plaques are present in the CA4 subregion of the hippocampus (F), whereas confluent looser deposits are characteristic in other subregions including CA1 (G) (Ab 5282; ×125). Cerebellum showed positivity in leptomeninges, leptomeningeal, and parenchymal blood vessels (H), with a perivascular plaque around the blood vessel in the granular cell layer (Ab 5282; ×30). Paraffin sections, hematoxylin counter stain.
The 10-nt duplication was present in six affected FDD family members (cases AII-1, AII-2, AIII-1, BII-2, BIII-1, and BIV-1) of the Danish kindred (Fig. 6). All of the patients tested so far are heterozygous, carrying one normal allele and one with the duplication. The 10-bp duplication was not observed in normal individuals (n = 78), patients with unrelated neurologic disorders (n = 42), or individuals with FBD (n = 8).
Discussion
Recent genetic and biochemical studies have shown that a number of genes are associated with the development of dementia in humans (7–10). In AD, at least four genes have been shown to be involved in the pathogenesis of the disorder. In early-onset familial AD, mutations in the amyloid precursor protein and in the presenilin-1 and presenilin-2 genes have been described (10). In the more common late-onset sporadic AD, the inheritance of the apolipoprotein ɛ4 allele constitutes a major risk factor (10). Very recently, we have identified a genetic defect in the BRI gene located on the long arm of chromosome 13 (13q14) (4, 6). The BRI gene is associated with the development of FBD, a disease that shares common features with AD (4, 7). The onset of FBD, characterized by progressive dementia, spastic paralysis, and cerebellar ataxia, normally occurs in the fifth decade of life. The pathological findings in these patients consist of widespread amyloid angiopathy in the cerebrum, cerebellum, and spinal cord, and the presence of mainly nonneuritic amyloid plaques and neurofibrillary tangles in the hippocampus (11). The major component of the fibrils in plaques and cerebrovascular amyloid is the ABri peptide, originated by a point mutation at codon 267 (T for A) in gene BRI (4). The mutation changes the normal stop codon into an arginine BRI (Stop-267→Arg), and as a result, the precursor protein extends 11 amino acids into the C terminus of the molecule, with the ABri protein being constituted by the last 34 amino acids of the mutated precursor.
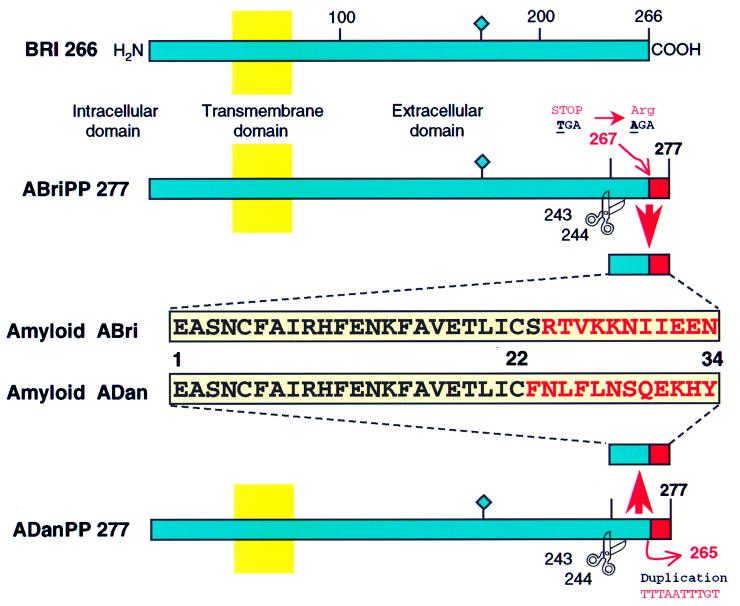
In the Danish kindred, a different genetic defect in gene BRI is associated with amyloid deposition and dementia, although with distinct phenotypic characteristics. Patients with FDD have a 10-nt duplication of the DNA sequence encoded between nucleotides 786 to 795 of the wild-type precursor cDNA sequence (795–796ins TTTAATTTGT). This DNA duplication produces the loss of the C-terminal serine (codon 266) causing a change in the reading frame that generates a completely different C-terminal sequence (last 12 amino acids) to that seen in the ABri peptide (Fig. 7). MS, amino acid sequence, and Western blot analysis data indicate that ADan deposited in the brain tissue of FDD patients is a peptide encoded by the last 34 amino acids of the abnormal precursor protein carrying the Danish mutation.
Figure 7.
Schematic representation of the amyloid precursor proteins and amyloid peptides in patients affected with FBD and FDD. The BRI gene codifies a putative single transmembrane-type II precursor protein of 266 amino acids. Different genetic defects in the BRI gene, namely a point mutation in FBD (Stop-267→Arg) and a 10-nt duplication (795–796ins TTTAATTTGT) in FDD, originate the two precursor molecules ABriPP and ADanPP, respectively. Although originated by different genetic defects in the BRI gene, both mutated precursors have 277 amino acids. The scissors indicate the cleavage site of the precursor molecules between codons 243 (Arg) and 244 (Glu) that releases the ABri and ADan peptides. Both amyloids have identical N-terminal amino acid sequence (first 22 amino acids) and a completely different C-terminal sequence (last 12 amino acids). The diamond indicates the single glycosylation site at position 170.
ADan and ABri peptides have a number of common features, although they originate from different genetic defects in the BRI gene. First, the amyloid precursor proteins in the British kindred (ABriPP) and in the Danish kindred (ADanPP) have the same length of 277 amino acids (Fig. 7). Second, ABri and ADan peptides also have the same length and share identical N-terminal amino acid sequence (first 22 residues), suggesting that these molecules are generated by the same proteolytic mechanism. Very recently, it has been suggested that furin, a secretory pathway endoprotease, may be involved in the processing of the BRI precursor protein (12), because the sequence KGIQKR↓, located immediately before the ABri and ADan N terminus, matches the consensus recognition sequence for proprotein convertases that belong to the subtilisin superfamily of calcium-dependent serine endoproteases (13). Third, ADan and ABri exhibit heterogeneity at the N- and C-terminal ends, as observed by MS analysis. The vast majority of the amyloid subunits are composed of the intact amyloid peptide featuring pyroglutamate at position one (ref. 4 and unpublished observations). Peptides starting at position 3 of both amyloids, representing a small portion of the total amyloid isolated, were detected by MS and direct amino acid sequence analysis, further suggesting the presence of similar degradation pathways. Fourth, the brain lesions in FDD are neuropathologically similar although not identical to the lesions observed in patients with FBD (11). In both conditions, there is severe amyloid angiopathy in the cerebrum and cerebellum with extensive hippocampal plaques including nonneuritic plaques, some resembling the cotton wool plaques of familial AD with PS1 Δ9 mutation (14) and neurofibrillary tangles. However, there are significant differences between the two diseases. In FDD the hippocampal deposits are mainly Congo-red negative, suggesting that the protein deposited is predominantly in a nonfibrillary structure. Furthermore, in the three cases with FDD, Aβ immunoreactivity was observed, mainly in a perivascular position. Further differences include that Danish patients present with early cataracts followed by hearing impairment 10 years later (1–3). Eye involvement has also been reported in a number of other forms of hereditary amyloidosis, such as those caused by mutations in the gelsolin gene (Asp-187→Asn) (15) and TTR gene (Val-30, Ile-33, Ser-84, Asn-84, and Cys-114 variants) (16, 17), but the presence of retinal lesions and cataracts concomitantly with deafness clearly distinguishes the Danish kindred from all other familial conditions.
Because FDD combines severe vascular amyloid, perivascular, and parenchymal plaques, as well as neurofibrillary tangle formation, its study and the development of animal models of the disease may provide useful information leading to the understanding of the relationship between cerebrovascular amyloidogenesis and neurodegeneration.
Acknowledgments
We thank Ms. Tammaryn Johnson for expert technical assistance with the immunostainings. This work was supported by National Institutes of Health grants AG08721, AG05891 (Merit Award), and AG10953 (Lead Award) to B.F. J.G. is the recipient of a New Investigator Development Award grant from the American Heart Association (New York City affiliate).
Abbreviations
- FDD
Familial Danish dementia
- ADan
Danish amyloid
- ABri
British amyloid
- AD
Alzheimer's disease
- FBD
familial British dementia
- Aβ
β-amyloid protein
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF246221).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080076097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080076097
References
- 1.Strömgrem E, Dalby A, Dalby M, Ranheim B. Acta Neurol Scand. 1970;46,(Suppl. 43):97–98. doi: 10.1111/j.1600-0404.1970.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 2.Strömgrem E. In: Handbook of Clinical Neurology. Vinken P J, Bruyn G W, editors. Amsterdam: Elsevier; 1981. pp. 150–152. [Google Scholar]
- 3.Plant G, Esiri M M. In: The Neuropathology of Dementia. Esiri M M, Morris J H, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 260–276. [Google Scholar]
- 4.Vidal R, Frangione B, Rostagno A, Mead S, Révész T, Plant G, Ghiso J. Nature (London) 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 5.Deleersnijder W, Hong G, Cortvrindt R, Poirier C, Tylzanowski P, Pittois K, Van Marck E, Merregaert J. J Biol Chem. 1996;271:19475–19482. doi: 10.1074/jbc.271.32.19475. [DOI] [PubMed] [Google Scholar]
- 6.Pittois K, Deleersnijder W, Merregaert J. Gene. 1998;217:141–149. doi: 10.1016/s0378-1119(98)00354-0. [DOI] [PubMed] [Google Scholar]
- 7.Price D. Nature (London) 1999;399:A3–A5. doi: 10.1038/399a003. [DOI] [PubMed] [Google Scholar]
- 8.Vidal R, Garzuly F, Budka H, Lalowski M, Linke R, Brittig F, Frangione B, Wisniewski T. Am J Pathol. 1996;148:361–366. [PMC free article] [PubMed] [Google Scholar]
- 9.Ghetti B, Piccardo P, Spillantini M,G, Ichimiya Y, Porro M, Perini F, Kitamoto T, Tateishi J, Seiler C, Frangione B, et al. Proc Natl Acad Sci USA. 1996;93:744–748. doi: 10.1073/pnas.93.2.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lendon C, Ashall F, Goate A. J Am Med Assoc. 1997;277:825–831. [PubMed] [Google Scholar]
- 11.Plant G T, Révész T, Barnard R O, Harding A E, Gautier-Smith P C. Brain. 1990;113:721–747. doi: 10.1093/brain/113.3.721. [DOI] [PubMed] [Google Scholar]
- 12.Kim S-H, Wang R, Gordon D, Bass J, Steiner D, Lynn D, Thinakaran G, Meredith S, Sisodia S. Nat Neurosci. 1999;2:984–988. doi: 10.1038/14783. [DOI] [PubMed] [Google Scholar]
- 13.Zhou A, Webb G, Zhu X, Steiner D F. J Biol Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 14.Crook R, Verkkoniemi A, Perez-Tur J, Metha N, Baker M, Houlden H, Farrer M, Hutton M, Lincoln S, Hardy J, et al. Nat Med. 1998;4:452–455. doi: 10.1038/nm0498-452. [DOI] [PubMed] [Google Scholar]
- 15.Ghiso J, Haltia M, Prelli F, Novello J, Frangione B. Biochem J. 1990;272:827–830. doi: 10.1042/bj2720827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen R B, Goren H, Cohen M, Richardson L, Tresser N, Lynn A, Gali M, Estes M, Gambetti P. Ann Neurol. 1997;41:307–313. doi: 10.1002/ana.410410305. [DOI] [PubMed] [Google Scholar]
- 17.Murakami T, Uchino M, Ando M. Pathol Int. 1995;45:1–9. doi: 10.1111/j.1440-1827.1995.tb03373.x. [DOI] [PubMed] [Google Scholar]