Abstract
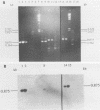
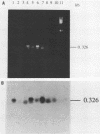
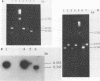
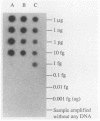
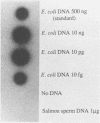
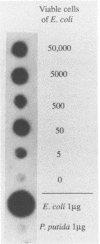
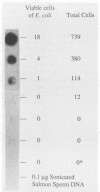
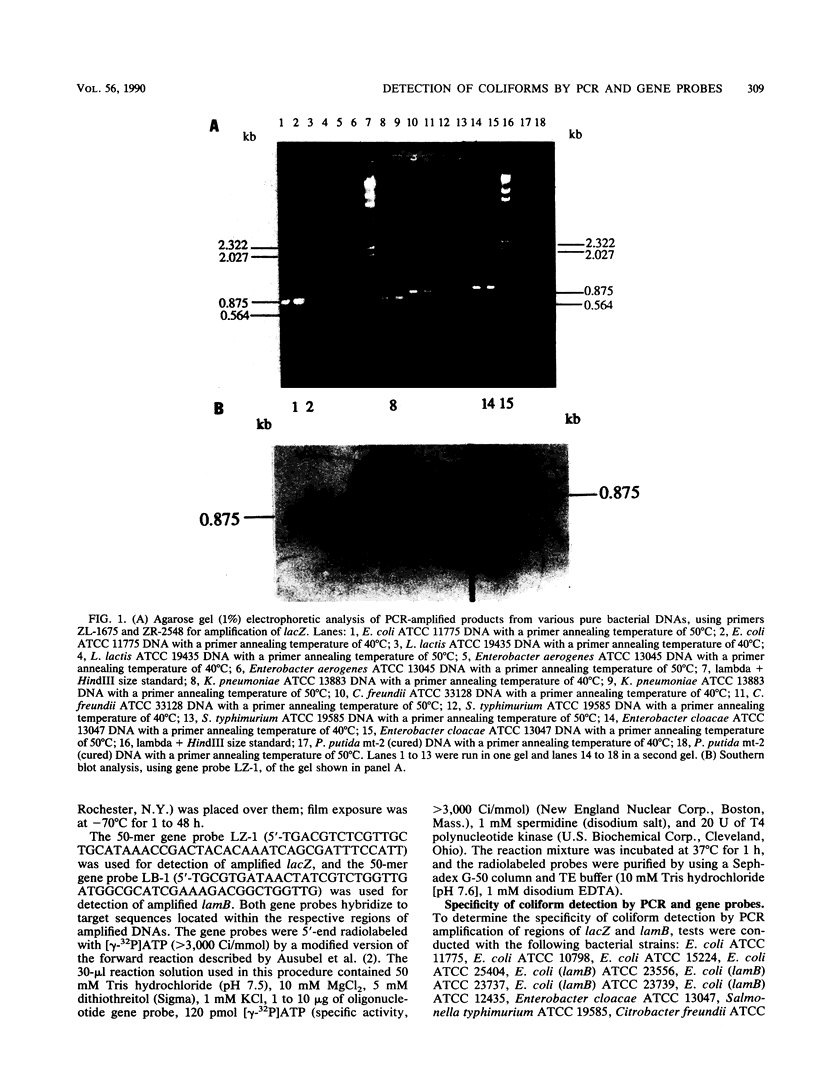
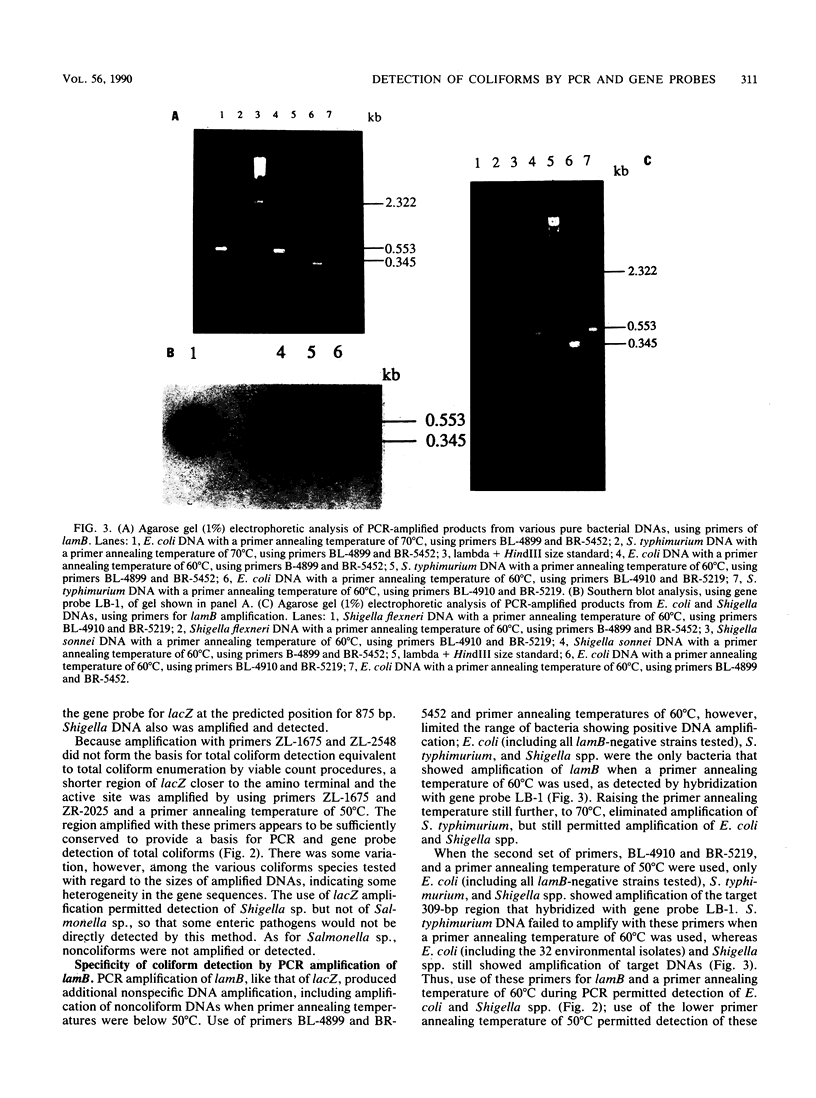
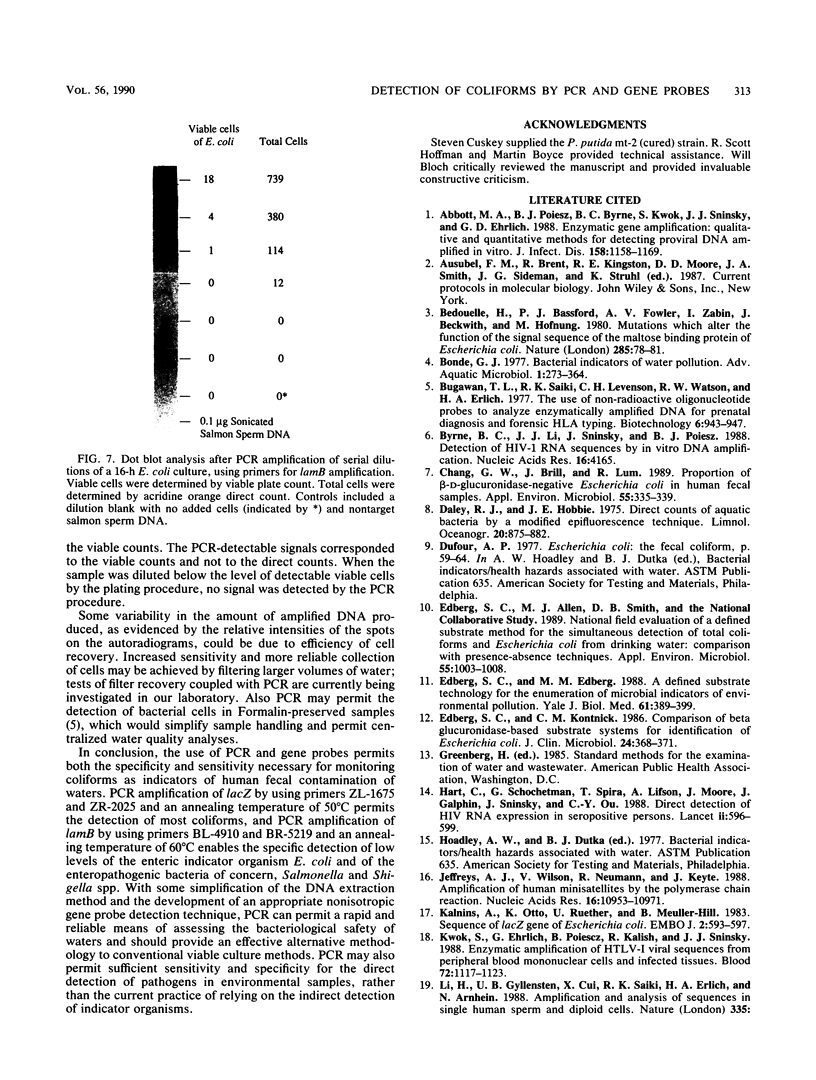
Polymerase chain reaction (PCR) amplification and gene probe detection of regions of two genes, lacZ and lamB, were tested for their abilities to detect coliform bacteria. Amplification of a segment of the coding region of Escherichia coli lacZ by using a PCR primer annealing temperature of 50 degrees C detected E. coli and other coliform bacteria (including Shigella spp.) but not Salmonella spp. and noncoliform bacteria. Amplification of a region of E. coli lamB by using a primer annealing temperature of 50 degrees C selectively detected E. coli and Salmonella and Shigella spp. PCR amplification and radiolabeled gene probes detected as little as 1 to 10 fg of genomic E. coli DNA and as a few as 1 to 5 viable E. coli cells in 100 ml of water. PCR amplification of lacZ and lamB provides a basis for a method to detect indicators of fecal contamination of water, and amplification of lamB in particular permits detection of E. coli and enteric pathogens (Salmonella and Shigella spp.) with the necessary specificity and sensitivity for monitoring the bacteriological quality of water so as to ensure the safety of water supplies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Brill J., Lum R. Proportion of beta-D-glucuronidase-negative Escherichia coli in human fecal samples. Appl Environ Microbiol. 1989 Feb;55(2):335–339. doi: 10.1128/aem.55.2.335-339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Allen M. J., Smith D. B. National field evaluation of a defined substrate method for the simultaneous detection of total coliforms and Escherichia coli from drinking water: comparison with presence-absence techniques. Appl Environ Microbiol. 1989 Apr;55(4):1003–1008. doi: 10.1128/aem.55.4.1003-1008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Edberg M. M. A defined substrate technology for the enumeration of microbial indicators of environmental pollution. Yale J Biol Med. 1988 Sep-Oct;61(5):389–399. [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Kontnick C. M. Comparison of beta-glucuronidase-based substrate systems for identification of Escherichia coli. J Clin Microbiol. 1986 Sep;24(3):368–371. doi: 10.1128/jcm.24.3.368-371.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C., Schochetman G., Spira T., Lifson A., Moore J., Galphin J., Sninsky J., Ou C. Y. Direct detection of HIV RNA expression in seropositive subjects. Lancet. 1988 Sep 10;2(8611):596–599. doi: 10.1016/s0140-6736(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Ehrlich G., Poiesz B., Kalish R., Sninsky J. J. Enzymatic amplification of HTLV-I viral sequences from peripheral blood mononuclear cells and infected tissues. Blood. 1988 Oct;72(4):1117–1123. [PubMed] [Google Scholar]
- Loche M., Mach B. Identification of HIV-infected seronegative individuals by a direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988 Aug 20;2(8608):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Olive D. M., Atta A. I., Setti S. K. Detection of toxigenic Escherichia coli using biotin-labelled DNA probes following enzymatic amplification of the heat labile toxin gene. Mol Cell Probes. 1988 Mar;2(1):47–57. doi: 10.1016/0890-8508(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Rayfield M., De Cock K., Heyward W., Goldstein L., Krebs J., Kwok S., Lee S., McCormick J., Moreau J. M., Odehouri K. Mixed human immunodeficiency virus (HIV) infection in an individual: demonstration of both HIV type 1 and type 2 proviral sequences by using polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1170–1176. doi: 10.1093/infdis/158.6.1170. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Ou C. Y., Jones W. K. Polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1154–1157. doi: 10.1093/infdis/158.6.1154. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]