Abstract
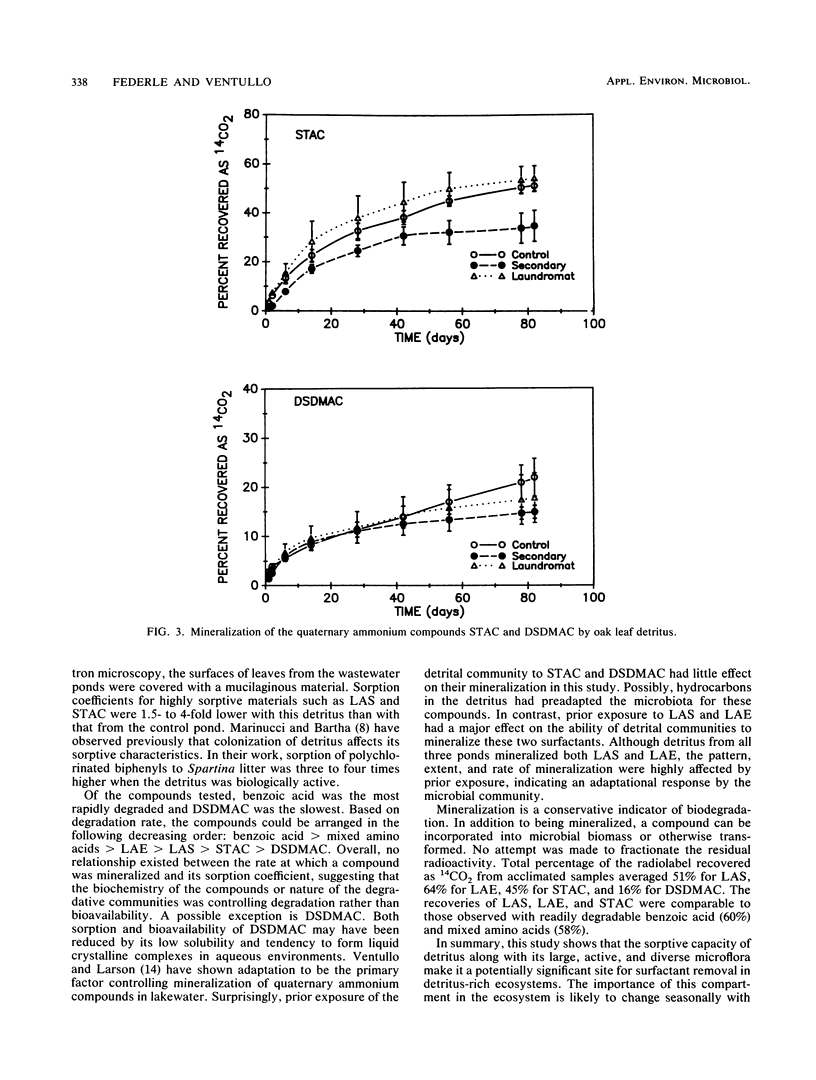
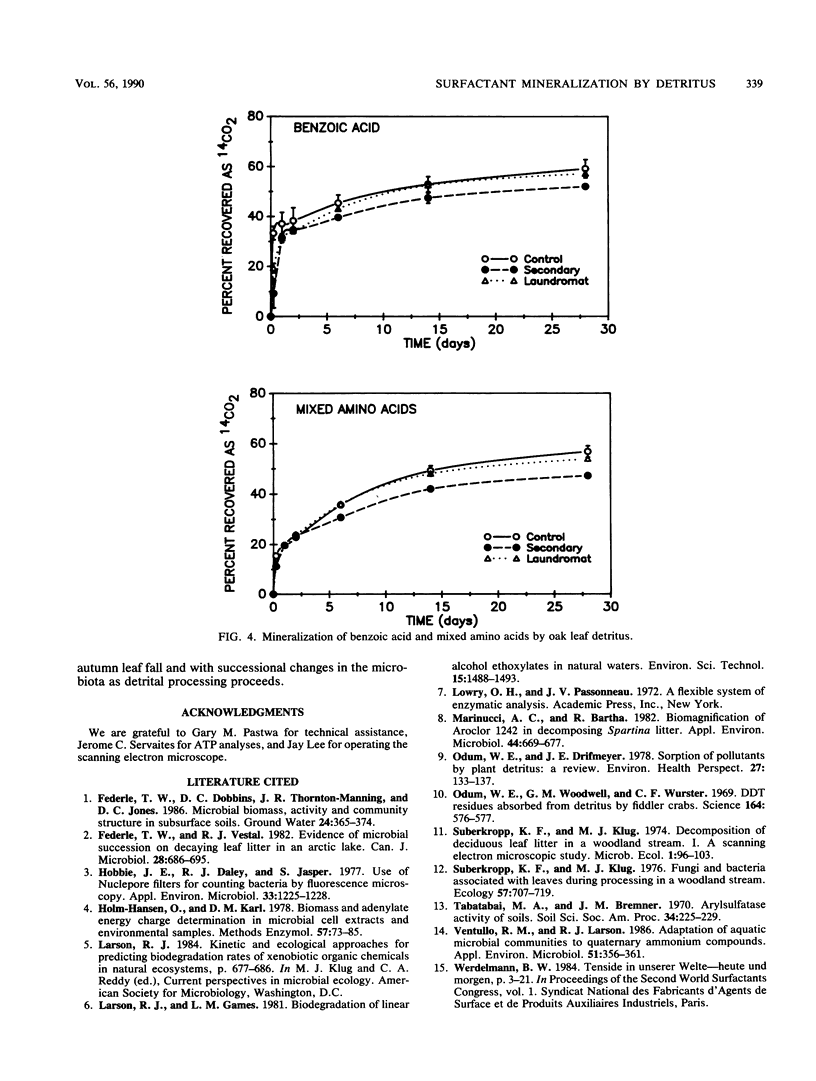
In wetlands and canopied bodies of water, plant detritus is an important source of carbon and energy. Detrital materials possess a large surface area for sorption of dissolved organics and are colonized by a large and diverse microbiota. To examine the biodegradation of surfactants by these microorganisms, submerged oak leaves were obtained from a laundromat wastewater pond, its overflow, and a pristine control pond. Leaves were cut into disks and incubated in sterile water amended with 50 μg of 14C-labeled linear alkylbenzene sulfonate (LAS), linear alcohol ethoxylate, stearyltrimethyl ammonium chloride, distearyldimethyl ammonium chloride, benzoic acid, or mixed amino acids per liter. Sorption of the test compounds to the detritus and evolution of 14CO2 were followed with time. All of the compounds sorbed to the detritus to various degrees, with LAS and stearyltrimethyl ammonium chloride the most sorptive and benzoic acid the least. All compounds were mineralized without a lag. With leaves from the laundromat wastewater pond, half-lives were 12.6 days for LAS, 8.4 days for linear alcohol ethoxylate, 14.2 days for stearyltrimethyl ammonium chloride, 1.0 days for benzoic acid, and 2.7 days for mixed amino acids. Mineralization of LAS and linear alcohol ethoxylate by control pond leaves was slower and exhibited an S-shaped rather than a typical first-order pattern. This study shows that detritus represents a significant site of surfactant removal in detritus-rich systems.
Full text
PDF

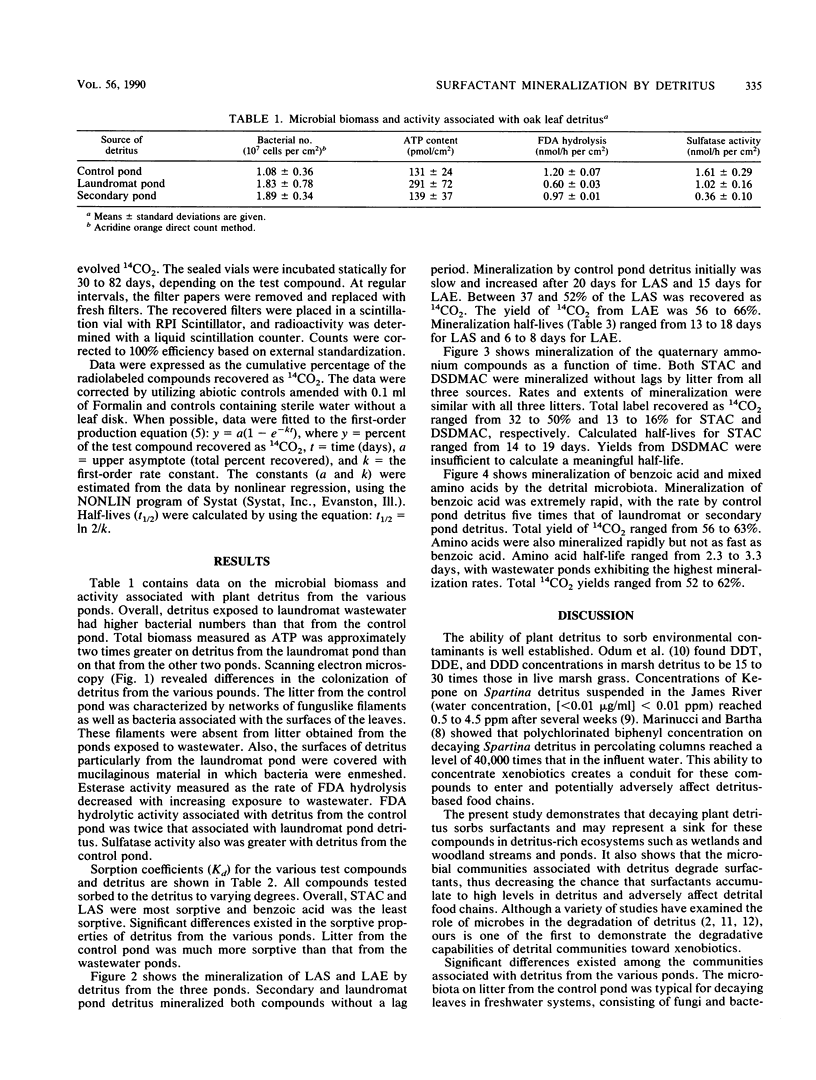
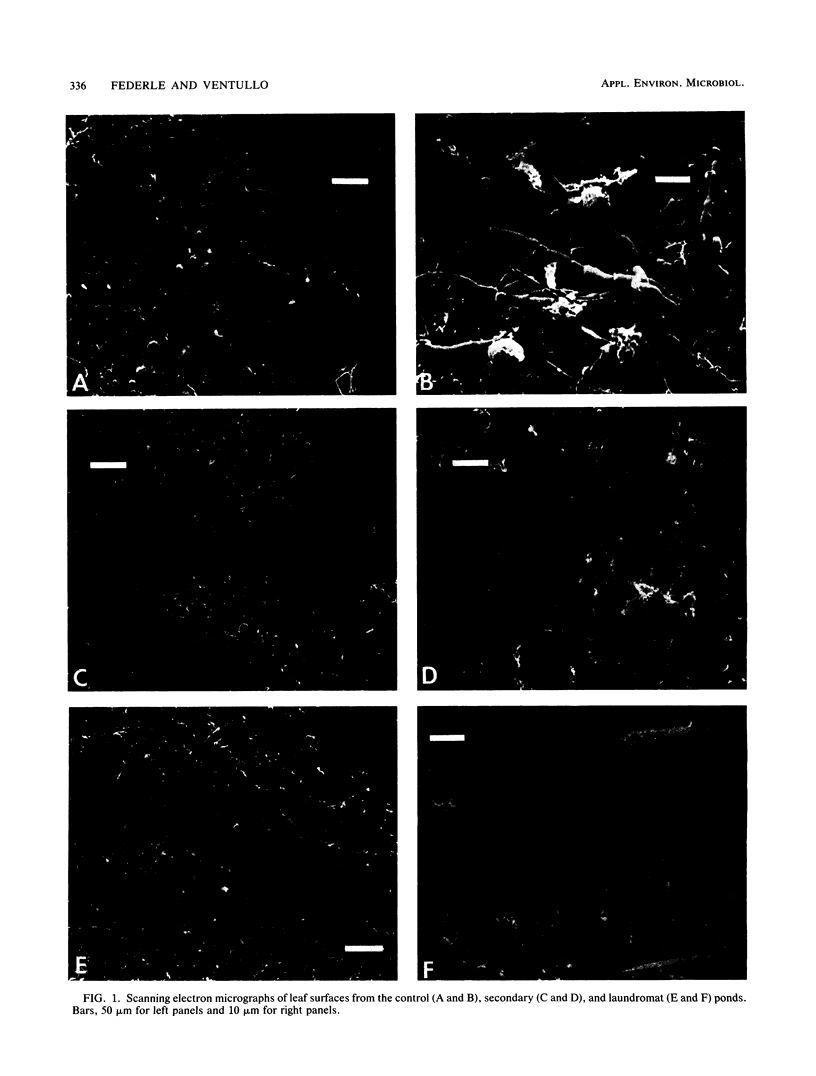
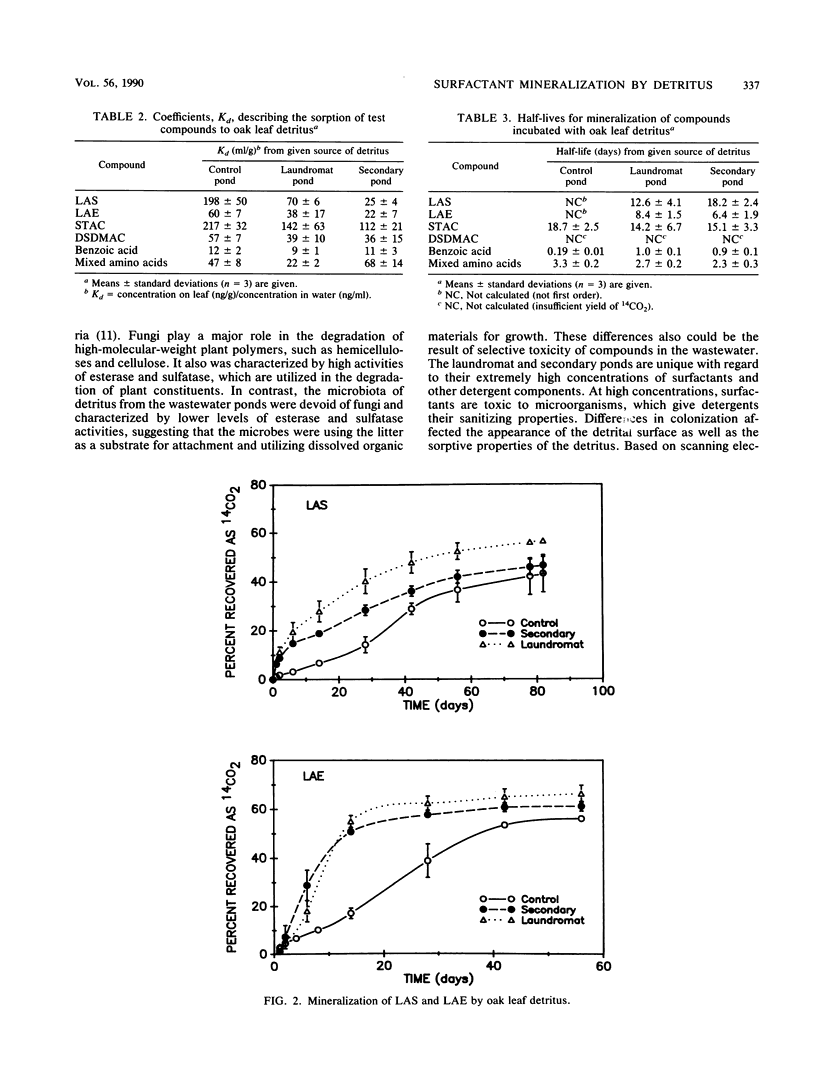
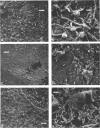
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Biomagnification of aroclor 1242 in decomposing spartina litter. Appl Environ Microbiol. 1982 Sep;44(3):669–677. doi: 10.1128/aem.44.3.669-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum W. E., Drifmeyer J. E. Sorption of pollutants by plant detritus: a review. Environ Health Perspect. 1978 Dec;27:133–137. doi: 10.1289/ehp.7827133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum W. E., Woodwell G. M., Wurster C. F. DDT Residues Absorbed from Organic Detritus by Fiddler Crabs. Science. 1969 May 2;164(3879):576–577. doi: 10.1126/science.164.3879.576. [DOI] [PubMed] [Google Scholar]
- Ventullo R. M., Larson R. J. Adaptation of aquatic microbial communities to quaternary ammonium compounds. Appl Environ Microbiol. 1986 Feb;51(2):356–361. doi: 10.1128/aem.51.2.356-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]