Abstract
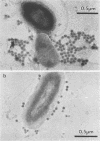
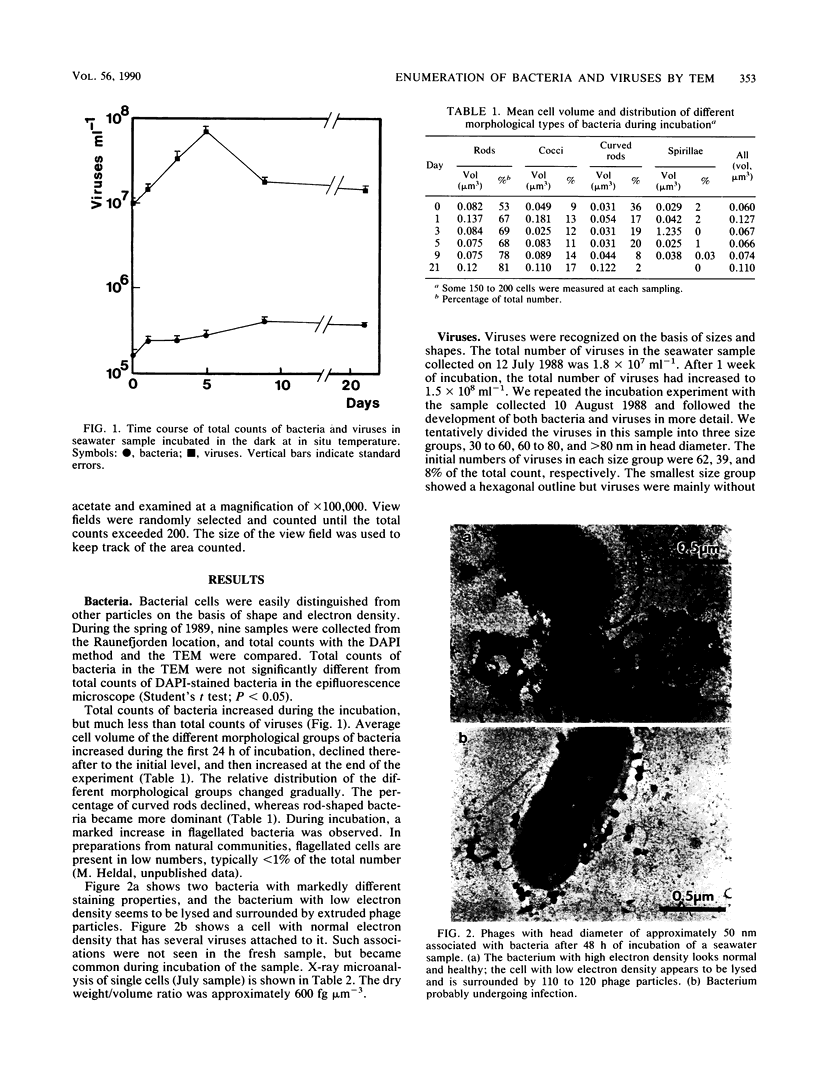
Bacteria and virus particles were harvested from water samples by ultracentrifugation directly onto Formvar-coated electron microscopy grids and counted in a transmission electron microscope. With this technique, we have counted and sized bacteria and viruses in marine water samples and during laboratory incubations. By X-ray microanalysis, we could determine the elemental composition and dry-matter content of individual bacteria. The dry weight/volume ratio for the bacteria was 600 fg of dry weight microns-3. The potassium content of the bacteria was normal compared with previous estimates from other bacterial assemblages; thus, this harvesting procedure did not disrupt the bacterial cells. Virus particles were, by an order of magnitude, more abundant than bacteria in marine coastal waters. During the first 5 to 7 days of incubation, the total number of viruses increased exponentially at a rate of 0.4 day-1 and thereafter declined. The high proliferation rate suggests that viral parasitism may affect mortality of bacteria in aquatic environments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G. Bacterial biovolume and biomass estimations. Appl Environ Microbiol. 1985 Jun;49(6):1488–1493. doi: 10.1128/aem.49.6.1488-1493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielowski J., Kłapcińska B. Bioaccumulation of Germanium by Pseudomonas putida in the Presence of Two Selected Substrates. Appl Environ Microbiol. 1986 May;51(5):1099–1103. doi: 10.1128/aem.51.5.1099-1103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert D. L., Paynter M. J. Technique for determining total bacterial virus counts in complex aqueous systems. Appl Environ Microbiol. 1980 Jan;39(1):253–260. doi: 10.1128/aem.39.1.253-260.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldal M., Norland S., Tumyr O. X-ray microanalytic method for measurement of dry matter and elemental content of individual bacteria. Appl Environ Microbiol. 1985 Nov;50(5):1251–1257. doi: 10.1128/aem.50.5.1251-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krambeck C., Krambeck H. J., Overbeck J. Microcomputer-assisted biomass determination of plankton bacteria on scanning electron micrographs. Appl Environ Microbiol. 1981 Jul;42(1):142–149. doi: 10.1128/aem.42.1.142-149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Fuhrman J. A. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton. Appl Environ Microbiol. 1987 Jun;53(6):1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieracki M. E., Johnson P. W., Sieburth J. M. Detection, enumeration, and sizing of planktonic bacteria by image-analyzed epifluorescence microscopy. Appl Environ Microbiol. 1985 Apr;49(4):799–810. doi: 10.1128/aem.49.4.799-810.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]