Abstract
The biochemical and behavioral effects of a nonpeptidic, selective, and brain-penetrant agonist at the ORL1 receptor are reported herein. This low molecular weight compound {(1S,3aS)-8- (2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza- spiro[4.5]decan-4-one} has high affinity for recombinant human ORL1 receptors and has 100-fold selectivity for ORL1 over other members of the opioid receptor family. It is a full agonist at these receptors and elicits dose-dependent anxiolytic-like effects in a set of validated models of distinct types of anxiety states in the rat (i.e., elevated plus-maze, fear-potentiated startle, and operant conflict). When given systemically, the compound has an efficacy and potency comparable to those of a benzodiazepine anxiolytic such as alprazolam or diazepam. However, this compound is differentiated from a classical benzodiazepine anxiolytic by a lack of efficient anti-panic-like activity, absence of anticonvulsant properties, and lack of effects on motor performance and cognitive function at anxiolytic doses (0.3 to 3 mg/kg i.p.). No significant change in intracranial self-stimulation performance and pain reactivity was observed in this dose range. Higher doses of this compound (≥10 mg/kg) induced disruption in rat behavior. These data confirm the notable anxiolytic-like effects observed at low doses with the orphanin FQ/nociceptin neuropeptide given locally into the brain and support a role for orphanin FQ/nociceptin in adaptive behavioral fear responses to stress.
The ORL1 orphan receptor was identified from a human cDNA library on the basis of close homology (≈65% in the transmembrane domains) with the μ-, δ-, and κ-opioid receptors (1, 2). Classical opioid ligands do not bind to ORL1, but orphanin FQ/nociceptin (OFQ/N), a 17-amino acid neuropeptide purified from brain extracts, was found to be the natural ligand of the G protein-coupled receptor ORL1 (3, 4). OFQ/N, its precursor peptide, and its receptor ORL1 are located in corticolimbic regions involved in the integration of the emotional components of fear and stress as well as in the spinal cord, with a pattern distinct from that of opioid peptides and receptors in rodents (5–9). The expression of OFQ/N or its receptor in the amygdaloid complex, septohippocampal region, periaqueductal gray matter, locus coeruleus, and dorsal raphe nucleus suggests that major brain neuronal systems may be sensitive to the action of OFQ/N. Such sensitivity has widespread implications for many aspects of behavior including arousal, attention, neuroendocrine control, fear, and anxiety (10). In brain slices, OFQ/N has potent inhibitory actions on neurons in the dorsal raphe nucleus, the locus coeruleus, the periaqueductal gray matter, and the amygdala (11–14). In general, OFQ/N plays an inhibitory role on synaptic transmission in the central nervous system and thereby may contribute to a reduction in responsiveness to stress. When given intracerebroventricularly to rodents, OFQ/N reduces elementary stress-induced physiological responses such as analgesia (15) but also attenuates elaborate behavioral fear responses elicited when animals are exposed to stressful/anxiogenic situations (16, 17). When given locally into the periaqueductal gray matter, it suppresses cingulate cortex-elicited vocalizations in the guinea pig (18). OFQ/N has also been found to play a direct role on pain perception, but despite much study, the precise effects of OFQ/N on nociceptive sensitivity remain unclear (19, 20).
Besides a function on pain control, the role of OFQ/N in regulating fear responses to stress opens avenues for exploring the pathophysiology of stress-related illnesses such as anxiety and depressive disorders. Agonists at ORL1 receptors may offer interesting possibilities for selective normalization of stress-related neuronal dysfunctions with limited side effects. Herein, we report on the biochemical and behavioral effects of a nonpeptidic and brain-penetrant full agonist at the ORL1 receptor, Ro 64-6198 {(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one}. This compound has a high affinity for recombinant human ORL1 receptors, has ≈100-fold selectivity versus other receptors in the opiate family, is a full agonist, and elicits anxiolytic-like effects in several validated anxiety models of distinctive types of anxiety states (spontaneous and conditioned) in the rat.
Materials and Methods
Animals.
The experimental procedures used in this study received approval based on international guidelines and adherence to Swiss federal regulations on animal experimentation. Animals were acclimatized to the laboratory conditions for several days before the start of the experiments. They were housed under standard lab conditions (temperature 20 ± 2°C; relative humidity 50–60%; with ad libitum access to food and water on a 12-h light/12-h dark cycle from 6 a.m. to 6 p.m.). They were tested during their diurnal light phase between 8 a.m. and 1 p.m. A total of 701 male Sprague–Dawley or Wistar rats were used in this study.
Drugs.
The selective ORL1 agonist Ro 64-6198, alprazolam, and diazepam were synthesized in the Central Nervous System Medicinal Chemistry Group at Hoffmann–La Roche (Basel). Solutions were freshly prepared in physiological saline containing 0.3% Tween 80 and were administered i.p. (5 ml/kg injection volume) in a logarithmic dose escalation with a 30-min pretreatment time to allow for optimal absorption and distribution.
Elevated Plus-Maze Test.
This test is based on the natural aversion of rodents for open spaces and heights. It uses an elevated maze with two open and two closed arms; the time spent and the number of entries into open arms are indices of neophobic anxiety in animals (21). Details on the apparatus have been described (22). Number of entries, distance, and total time spent in the open arms were automatically measured over 5 min. Distance and number of closed arm entries were also recorded as measures of general activity. Animals falling off the maze were eliminated from the analysis. Forelimb grip strength was assessed subsequently by using a digital strain gauge connected to a triangular bar (2-mm diameter, 5 cm wide). For each animal, the maximum of three permissible readings was recorded as forelimb grip strength (in grams). Total duration of this test was 1 min. Naïve Sprague–Dawley rats (n = 256; 120–150 g) were used (n = 32 for each dose of each drug).
Fear-Potentiated Auditory Startle.
In the fear-potentiated startle procedure, a neutral stimulus such as light is repetitively paired with an aversive stimulus such as mild foot shock. When an animal is presented with loud acoustic stimuli, enhanced startle responses are elicited when the startle stimulus is preceded by the light (a classically conditioned increase in fear). Benzodiazepines and related compounds attenuate the response enhancement. Details of the method are described elsewhere (23). Startle response in rats was recorded by using auditory startle chambers. After a 95-dB habituation session in the dark, 10 randomly alternating acoustic stimuli of 90, 95, or 105 dB (50-ms duration) were given in the presence or absence of light. Startle response amplitudes for each trial type were averaged for each rat across the entire test session. Naïve Wistar rats (n = 128; 260–280 g) were used (n = 16 for each dose of each drug).
Operant Conflict Procedure.
In a modified Geller–Seifter conflict test, food-deprived animals are given the opportunity to press a bar to obtain a 45-mg food pellet in a test chamber (30 × 25 × 30 cm high) with a grid floor. Nonconflict sessions are periods where bar presses are reinforced with food. They alternate with conflict sessions in which bar press results in food delivery and simultaneous electric foot shock (scrambled 1.5-mA foot shock for 400 ms). The shock typically results in a selective inhibition of behavior. Benzodiazepines and related compounds attenuate the response inhibition (24, 25). Rats served as their own controls, received several drug treatments, and were exposed to one nonconflict and one conflict session each week. In the conflict sessions, treatment with vehicle alternated with test compound, and the total number of lever presses was measured during 1 h. Male well trained Wistar rats (n = 14; 200–250 g) were used.
Panic-Like Anxiety Test.
Acute and intense aversive effects are induced, in animals and humans, by dorsal periaqueductal gray matter stimulation. These aversive effects are reflected in animals by abrupt escape reactions accompanied by fear-characteristic autonomic changes. This model in the rat has been validated as realistically simulating particular aspects of panic anxiety with objective signs of symptomatic and predictive validity, and details of the method have been described (26). Stimulation threshold for dorsal periaqueductal gray matter self-interruption behavior was taken as a measure of sensitivity to the panic-like aversion induced by the stimulation. Latencies were recorded to evaluate drug effects on motor performance. A group of 31 well-trained Wistar rats (300–350 g) was used. Animals served as their own controls and received several drug treatments in a crossover design and counterbalanced order. At least 1 week separated two consecutive test drug injections, and a vehicle treatment separated drug injections from each other.
Motor Coordination and Balance.
The rotarod test situation consists of placing an animal on a horizontal metal rod (10 cm wide, 5 cm in diameter, 40 cm above the bench) rotating twice per minute. Mild sedation or myorelaxation translates into motor incoordination on the rotating rod, and animals fall off the bar. Time spent on the rotating rod is measured in seconds, and maximal cut-off time is 60 s. The traction test consists of forcing rats to grasp a horizontally strung wire (20 cm above the bench level, 2 mm in diameter, 20 cm long) with their forepaws. Various items are scored: the grasp reflex (score 1), body weight support (score 2), climb reflex (score 3), and escape (score 4); group scores are calculated by averaging individual scores. Total duration of these tests is 2 min. Naïve Sprague–Dawley rats (n = 72; 120–150 g) were used (n = 8–10 for each dose of each drug).
Epileptic Seizures.
Pentylenetetrazol (120 mg/kg i.p.) was used as a standard chemical convulsant eliciting a characteristic epileptic-like syndrome. Latencies to the first episode of limb tremors and clonic seizures, followed by generalized clonic-tonic convulsions, were measured for each animal. Animals were euthanized directly after the first episode of generalized clonic-tonic convulsions, which is the main pharmacodynamic endpoint considered in this study. Increase in latency or full protection against clonic or tonic seizures (30-min observation period) is considered as an index for anticonvulsant activity. Naïve Sprague–Dawley rats (n = 64; 160–180 g) were used (n = 8 for each dose of each drug).
Intracranial Self-Stimulation.
Test chambers consisted of Plexiglas boxes (30 × 25 × 25 cm) with a hole (2.5-cm diameter). Rats could interrupt a convergent light beam by poking their noses into the hole to trigger a rewarding intracranial electrical stimulation via an electrode stereotaxically implanted within the mesolimbic system at the level of the ventral tegmental area. Thresholds for self-stimulation behavior were determined as described (27). A group of 16 Wistar rats (300–350 g) were implanted for the study. Animals served as their own controls and received several drug treatments in a crossover design and counterbalanced order. At least 1 week separated two consecutive test drug injections, and a vehicle treatment separated drug injections from each other.
Anterograde Amnesia in a Passive Avoidance Task.
Passive avoidance training was carried out as described (28). In this paradigm, animals learn under vehicle or drug that they can avoid a 1.1-mA electric foot shock administered through the floor grid of the cage (40 × 31 × 29 cm) by remaining on a safe plastic platform (15 × 15 cm) covering the grid in one corner. Each rat received three training sessions to criterion given 30, 60, and 90 min after administration. At 6 h after injection, memory retention is tested by placing the animals on the platform. Failure to stay on the plastic platform voluntarily within the initial 1 min of the retention test or subsequently failing to resist being pushed onto the grid floor was judged as an avoidance failure and, thus, as evidence for amnesia. Naïve Wistar rats (n = 120; 100–120 g) were used (n = 30 for each dose of each drug).
Statistical Analysis.
Dose-effects were assessed by single factor (dose) analysis of variance. Where indicated by near-significant and significant F values, further a posteriori comparisons of individual doses versus vehicle effects were performed by using Bonferroni–Dunn statistics and a 5% significance level. Based on a priori information on possible nonnormal distributions, nonparametric statistics were used in the conflict and passive avoidance test. A Wilcoxon test was used to compare each dose to vehicle in the operant conflict study, and a two-tailed χ2 test was used for analyzing passive avoidance data. Descriptive statistics are means ± SEM.
Binding Assays.
Competitive binding displacement analyses were performed with membranes prepared from permanently transfected HEK293 cells expressing human ORL1 (hORL1) and 0.1 nM [leucyl-3H]OFQ. Competitive binding displacement analyses for opioid receptors were performed with membranes prepared from BHK cells transiently expressing human μ- (hμ-), human κ- (hκ-) or human δ- (hδ-)opioid receptors, by using Semliki forest virus vectors (29). [N-allyl-2, 3-3H]naloxone at 1.5 nM and 3 nM was used to label hμ- and hκ-receptors, respectively, and 0.3 nM of [Ile5,6-3H]deltorphin was used for hδ-receptors. The radioligands were from Amersham Pharmacia. Nonspecific binding was defined in the presence of 1 μM unlabeled OFQ (for hORL1), 10 μM naloxone (for hμ and hκ), or 10 μM deltorphin (for hδ). Average pKi values were generated from four independent experiments.
γ-[35S]thio-GTP (GTPγ35S) Binding Assay.
Agonist-mediated binding of GTPγ35S was investigated in 96-well plates by using a scintillation proximity assay, and membranes were prepared from cells expressing hORL1, hμ-, hκ-, or hδ-receptors. Binding was performed in the presence of membranes, 1 mg of wheat germ agglutinin scintillation proximity assay beads (Amersham Pharmacia), and either OFQ (10 μM to 0.1 nM) or synthetic compounds (100 μM to 0.1 nM). The reaction mixture was incubated on a shaker for 60 min at 22°C and finally read in a Top Counter (Packard).
cAMP Assay.
The inhibition of forskolin-mediated cAMP accumulation by OFQ and Ro 64-6198 was determined in 96-well plates. Briefly, 20,000 cells were incubated in Krebs–Ringer/Hepes-buffered solution (124 mM NaCl/5 mM KCl/1.25 mM MgSO4/1.5 mM CaCl2/1.25 mM KH2PO4/25 mM Hepes, pH 7.4) in the presence of 100 μM rolipram and 1 μM forskolin (both from Sigma) with increasing concentrations of agonists (10 pM to 100 nM) for 15 min at 37°C. Reactions were stopped by the addition of 0.12 ml of ice-cold ethanol and stored at −80°C for at least 4 h. The cAMP content was determined from the supernatant by using the Biotrak nonradioactive cAMP kit (Amersham Pharmacia) according to the manufacturer's instructions.
Results
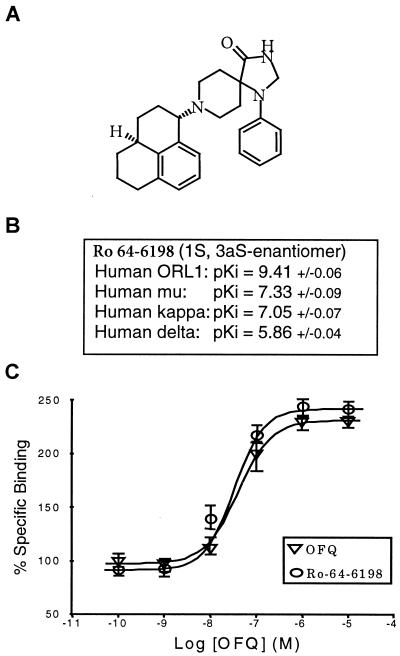
Ro 64-6198 competed with high affinity (pKi = 9.4) for [3H]OFQ binding to human ORL1 receptors (pKi = 9.0 for the OFQ/N peptide). Lower affinities were detected for hμ and hκ opioid receptors, conferring a relative selectivity of at least 100-fold over opioid receptors (Fig. 1). Ro 64-6198 had no significant affinity (IC50 > 1 μM) for 44 other receptor or channel binding sites in the brain but had micromolar affinity (IC50 ≈ 1 μM) for delta opioid, histamine H2, σ, dopamine D2 receptors, and sodium-site 2 channels. Ro 64-6198 was a full agonist at the human OFQ receptor when tested for its potency to stimulate GTPγ35S binding (pEC50 = 7.41; pEC50 = 7.37 for OFQ/N) and to inhibit forskolin-stimulated cAMP accumulation (pEC50 = 9.49 nM).
Figure 1.
Chemical structure of Ro 64-6198 (A), affinity values (± SEM) of Ro 64-6198 for recombinant human opioid receptor subtypes (B), and effects of Ro 64-6198 and OFQ/N on stimulation of GTPγ35S binding in vitro (C).
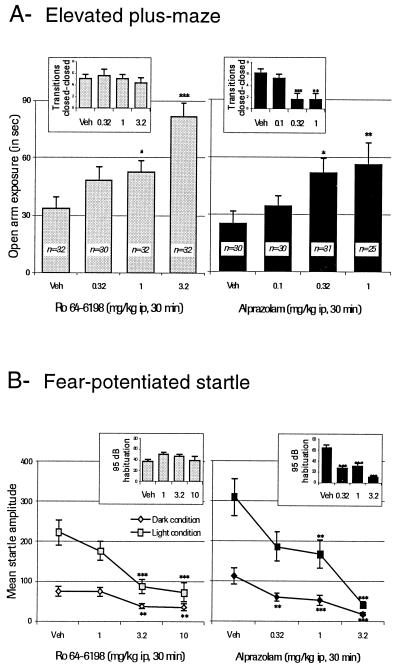
After i.p. administration in rats (0.3, 1, and 3.2 mg/kg versus vehicle; 30-min pretreatment), Ro 64-6198 increased exploration in an elevated plus-maze (Fig. 2). Increases were specifically observed for transitions, time spent, and distance moved (F = 10.23, F = 9.82, and F = 10.85, respectively; P < 0.001) in the open arm. Alprazolam also significantly increased these same parameters (F = 5.05, F = 3.86, and F = 3.61, respectively; P < 0.05). No statistically significant effects were detected on locomotion in the closed arms after treatment with Ro 64-6198 (F = 0.795; P > 0.05 on transitions; Fig. 2; F = 1.106; P > 0.05 on distance moved), whereas significant decreases were detected after treatment with alprazolam on transitions (F = 6.54; P = 0.0004; Fig. 2) and on distance moved in the closed arms (F = 10.85; P = 0.0001). After the plus-maze test, forced motor performance measured on grip strength remained intact and varied from 565 ± 7.6 g under vehicle to 548 ± 7.9 g after 3.2 mg/kg i.p. of Ro 64-6198 (P = 0.14; Bonferroni–Dunn). However, clear myorelaxation was detected after 1 mg/kg i.p. of alprazolam: seven animals fell off the maze, and grip strength decreased from 527.0 ± 11.8 g under vehicle to 460.1 ± 9.8 g (P = 0.001; Bonferroni–Dunn).
Figure 2.
Effect of Ro 64-6198 in comparison to alprazolam on exploration in the elevated plus-maze test (A) and in fear-potentiated startle in rats (B). For both tests, *, P < 0.05; **, P < 0.01; ***, P < 0.001 when compared with vehicle; Bonferroni–Dunn post hoc test for multiple comparisons. Veh, vehicle.
In the acoustic startle paradigm, the ORL1 agonist Ro 64-6198 (1, 3.2, and 10 mg/kg versus vehicle; 30-min pretreatment) attenuated startle responses (F = 8.26; P < 0.001; under light) measured during the test session (Fig. 2) without significantly impairing responses in the initial habituation trial (F = 1.134; P = 0.34). Alprazolam showed equivalent activity in attenuating startle responses (F = 12.82; P = <0.001; under light) but also significantly decreased responses in the habituation trial (F = 22.802; P < 0.001).
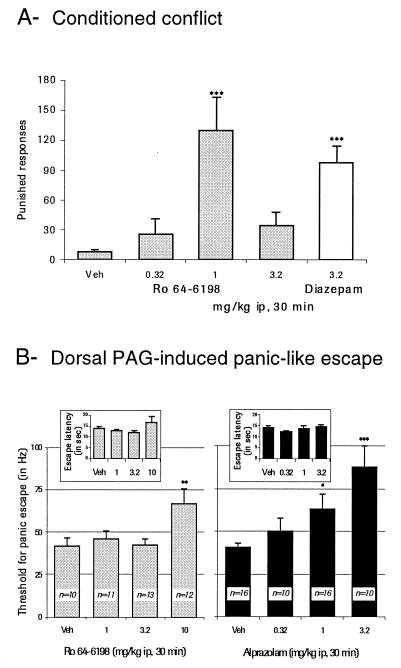
Ro 64-6198 injected i.p. (0.3, 1, and 3.2 mg/kg versus vehicle; 30-min pretreatment) was effective in increasing the rate of punished responding (F = 17.96; P = 0.0001) in a modified Geller–Seifter conflict procedure (Fig. 3). A typical bell-shaped dose-response relationship was observed, with the greatest effect seen at a dose of 1 mg/kg. Significant differences with vehicle were recorded after treatment with 3.2 mg/kg diazepam (P < 0.01; Wilcoxon).
Figure 3.
(A) Effect of Ro 64-6198 in comparison to diazepam in a conditioned conflict test in the rat (**, P < 0.01 when compared with vehicle; Wilcoxon test). (B) Effect of Ro 64-6198 in comparison to alprazolam on panic-like anxiety elicited by dorsal periaqueductal gray matter stimulation in the rat (*, P < 0.05; **, P < 0.01; ***, P < 0.001 when compared with vehicle; Bonferroni–Dunn post hoc test for multiple comparisons). Veh, vehicle.
No modifications in threshold for panic-like anxiety were detected under Ro 64-6198 treatment (Fig. 3) at 1 and 3.2 mg/kg versus vehicle (30-min pretreatment). Significant increases were recorded at 10 mg/kg (P = 0.0053; Bonferroni–Dunn), which also tended to increase self-interruption latency (Fig. 3). Alprazolam (0.3, 1, and 3.2 mg/kg versus vehicle; 30-min pretreatment) dose-dependently increased thresholds (F = 6.14; P = 0.0013) without significantly affecting latencies in this dose range (F = 0.972; P = 0.414).
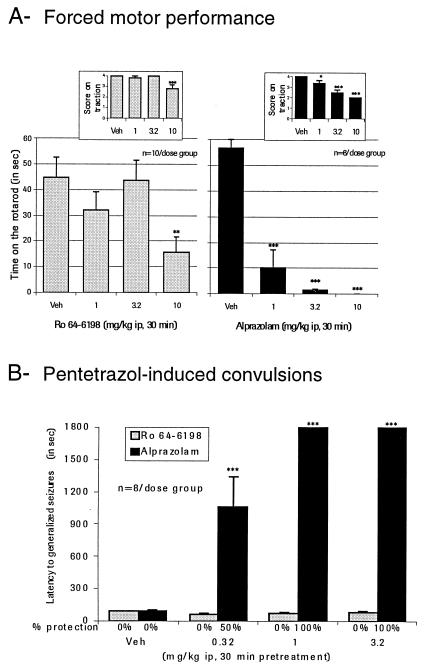
Significant impairment in forced motor performance was detected on rotarod and traction tests after treatment with 10 mg/kg Ro 64-6198 (Fig. 4; P = 0.006 and 0.001 versus vehicle, respectively; Bonferroni–Dunn test). Alprazolam was significantly active in reducing performance at 1 mg/kg i.p. and higher (F = 45.92; P = 0.0001; P = 0.0001 versus vehicle; Bonferroni–Dunn).
Figure 4.
Effect of Ro 64-6198 in comparison to alprazolam on forced motor performance in the rat (A) and on pentetrazol-induced convulsions in the rat (B). For both tests, *, P < 0.05; **, P < 0.01; ***, P < 0.001 when compared with vehicle; Bonferroni–Dunn post hoc test for multiple comparisons. Veh, vehicle.
In a model of chemically induced convulsions in the rat, no significant increase or decrease (F = 1.28; P = 0.30) in latency for the first episode of generalized clonic-tonic seizures (Fig. 4) was detected after Ro 64-6198 administration (0.3, 1, and 3.2 mg/kg versus vehicle; 30-min pretreatment). Alprazolam, however, was highly effective in delaying the occurrence of seizures (F = 32.84; P < 0.001) and in protecting the animals against convulsions induced by pentylenetetrazol.
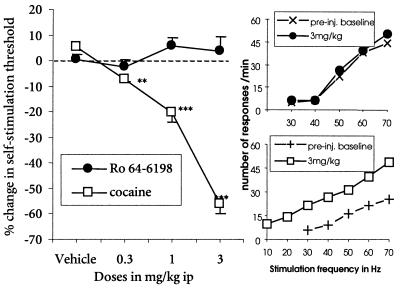
The ORL1 agonist Ro 64-6198 (0.3, 1, and 3.2 mg/kg versus vehicle; 30-min pretreatment) did not significantly modify (F = 0.79; P = 0.50) the threshold for self-stimulation in animals stereotaxically implanted with electrodes into the ventral tegmental area, in contrast to cocaine (Fig. 5). Performance on this test, measured as number of nose pokes per minute, was also not affected by Ro 64-6198 in the anxiolytic dose range but was enhanced by cocaine (Fig. 5).
Figure 5.
Effect of Ro 64-6198 in comparison to cocaine on intracranial self-stimulation thresholds in the rat (**, P < 0.01; ***, P < 0.001 when compared with vehicle; Bonferroni–Dunn post hoc test for multiple comparisons). pre-inj., preinjection.
Under the passive avoidance conditions used in this investigation (1 and 3.2 mg/kg versus vehicle; 30-min pretreatment), 3.3% of the rats injected with vehicle before training showed a retention deficit. The ORL1 agonist Ro 64-6198 produced a nonsignificant increase in the proportion of rats showing a retention deficit (23.3%) at 3.2 mg/kg i.p. (χ2 = 3.6; P > 0.05; n = 30), whereas alprazolam administered at 1 mg/kg i.p. significantly increased the proportion showing a retention deficit to 53.3% (χ2 = 16.1; P < 0.01; n = 30).
In a behavioral observation experiment, high doses of Ro 64-6198 (starting at 10 mg/kg i.p.) were found to interfere with normal behavioral performance in the rat. They induced an atypical syndrome composed of mixed ataxia, spontaneous jerks, and bouts of food intake.
Discussion
Ro 64-6198 has high affinity for recombinant human ORL1 receptors and 100-fold selectivity over other members of the opiate receptor family. Ro 64-6198 is a full agonist at these receptors and elicited positive effects in several validated models of distinct types of anxiety states in the rat. It increased open arm exploration in an elevated plus-maze test, augmented rates of punished responding in an operant conflict procedure, and decreased fear-potentiated responses in an acoustic startle paradigm. These effects are all indications of anxiolytic-like activity in the rat and are consistent with the anxiolytic-like effects previously reported after intracerebroventricular infusion of the OFQ/N peptide in several validated models of anxiety in rodents (16–18).
Unlike buspirone or selective serotonin reuptake inhibitors, the ORL1 agonist Ro 64-6198 induced effects in rats with amplitude comparable to that of conventional benzodiazepine anxiolytics such as alprazolam or diazepam in these models. Effects were very consistent and observed in different rat models of anxiety, along the lines of the principle that reliable effects on behavior must come from converging evidence obtained by different approaches. These included nonconditioned phobic-like anxiety in the plus-maze and conditioned emotional responses or operant responses such as those measured in the fear-potentiated startle and operant conflict procedures; all of these results indicate potential for anxiolytic effects in humans.
However, the ORL1 agonist was only marginally active in a test of panic-like anxiety in the rat in which alprazolam is significantly efficacious. This observation is consistent with data of a previous OFQ/N study suggesting that the peptide may not play a major role in the modulation of panic-related behavior (17). In this study, by using a defense test battery in which mice are confronted with a natural threat, OFQ/N did not decrease a measure of avoidance distance that is highly sensitive to treatment with antipanic compounds. Thus, clear differences also exist between ORL1 agonists, OFQ/N, and conventional benzodiazepines. Unlike alprazolam, Ro 64-6198 is also clearly devoid of anticonvulsant properties, does not significantly impair memory functions, and does not affect sensorimotor functions in the dose range found to be active in anxiety tests.
Higher doses of Ro 64-6198 (10 mg/kg i.p.) were found to disrupt rat behavior in forced motor performance and panic escape latencies. These higher doses also induced an atypical syndrome (i.e., made of ataxia, jerks, and bouts of food intake) likely to relate to the high concentrations rapidly reached in the brain with this compound. Indeed, despite a low oral bioavailability (≈4%), Ro 64-6198 has excellent brain penetration after parenteral injection (up to 1,000 ng/g reached 15 min after injection of 10 mg/kg i.p., with a relatively slow elimination; data not shown). Side effects seen at high doses might be connected to activity at several binding sites with micromolar affinity for Ro 64-6198, including ORL1 receptors. Indeed, OFQ/N itself was found to interfere with regular sensorimotor function at high doses (>3 nmol intracerebroventricularly for OFQ/N), as do conventional benzodiazepine (≥1 mg/kg i.p. for alprazolam). Because no selective ORL1 antagonist is available yet, the mechanisms underlying the behavioral syndrome seen at high doses of Ro 64-6198 are unclear at the moment. Pretreatment with naloxone did not significantly modify the behavioral syndrome, suggesting that opiate receptors are not involved; however, a contribution of σ, histamine H2, dopamine D2, and Na+ channel site 2 binding sites cannot be totally excluded.
Naloxone was also unable to reverse the effects of Ro 64-6198 in the elevated plus-maze test (data not shown), suggesting that opioid receptors are not involved in mediating its anxiolytic-like effects. Ro 64-6198 given alone at anxiolytic doses did not modify pain perception measured in a tail flick assay in the rat, nor did it induce allodynia in a tactile sensitivity test with Von Frey hairs (data not shown). Thus, despite its structural relation to the opioid system, OFQ/N does not seem to share functional relationships with opioids. OFQ/N (0.1–100 nmol) does not produce conditioned place preference or aversion in rats, suggesting that OFQ/N lacks the motivational effects of μ- and δ-opioid receptor agonists, which produce conditioned place preference when administered at behaviorally active doses (30). OFQ/N also fails to affect heroin self-administration in rats, suggesting that OFQ/N does not affect the rewarding value of heroin (31). Finally, the ORL1 agonist Ro 64-6198, in sharp contrast to cocaine, did not significantly modify ventral tegmental self-stimulation, suggesting that this new class of drugs is devoid of potential for nonmedical use and dependence characteristic of many abused psychostimulants.
Mice were more sensitive to the disruptive effects of Ro 64-6198 than were rats, and robust anxiolytic-like effects were consequently more difficult to reveal with Ro 64-6198 in this species (data not shown). Intracerebroventricular OFQ/N was significantly active in mouse and rat anxiety models (16, 17), suggesting that the species difference seen in responsiveness to Ro 64-6198 probably relates to intrinsic properties of the compound. Very high concentrations rapidly reached in brain and micromolar affinity of Ro 64-6198 for σ, histamine H2, dopamine D2, and Na+ channel site 2 binding sites are likely reasons for its disruptive effects. Different subtypes of OFQ receptors with different receptor reserves and organ and species distributions also possibly mediate the diverse effects of OFQ/N. Data obtained in ORL1-deficient mice suggest that ORL1 is the only target for OFQ/N in the mouse brain (32). However, a number of splice variants have been identified and have different regional distribution in the mouse brain (33, 34). In humans, polymorphism in ORL1 may associate with different types of stress-related disorders including various kinds of anxiety or depressive disorders. The biological significance of ORL1 polymorphs and of ORL1 receptor subtypes clearly remains to be established. The availability of a selective ORL1 agonist is an important step to facilitate the elucidation of the multiple functions of ORL1 in the brain. It also opens avenues for exploring the pathophysiology of anxiety disorders and for discovery of innovative anxiolytics.
Acknowledgments
We wish to thank Patrick Biry, Martine Kapps, Martine Maco, Jacqueline Higelin, Sandra Mueller, Philipp Oberli, Daniel Wehrli, and Laurent Gaudet for their skillful technical assistance.
Abbreviations
- OFQ/N
orphanin FQ/nociceptin
- Ro 64-6198
(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one
- GTPγ35S
γ-[35S]thio-GTP
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090514397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090514397
References
- 1.Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, Caput D, Vassart G, Meunier J-C. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 2.Bunzow J R, Saez C, Mortrud M, Bouvier C, Williams J T, Low M, Grandy D K. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 3.Reinscheid R K, Nothacker H P, Bourson A, Ardati A, Henningsen R A, Bunzow J R, Grandy D K, Langen H, Monsma F J, Jr, Civelli O. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 4.Meunier J C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J L, Guillemot J C, Ferrara P, Monsarrat B, et al. Nature (London) 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 5.Houtani T, Nishi M, Takeshima H, Nukada T, Sugimoto T. Biochem Biophys Res Commun. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- 6.Schulz S, Schreff M, Nuss D, Gramsch C, Hollt V. NeuroReport. 1996;7:3021–3025. doi: 10.1097/00001756-199611250-00045. [DOI] [PubMed] [Google Scholar]
- 7.Florin S, Leroux-Nicollet I, Meunier J C, Costentin J. Neurosci Lett. 1997;230:33–36. doi: 10.1016/s0304-3940(97)00470-9. [DOI] [PubMed] [Google Scholar]
- 8.Shimohira I, Tokuyama S, Himeno A, Niwa M, Ueda H. Neurosci Lett. 1997;237:113–116. doi: 10.1016/s0304-3940(97)00807-0. [DOI] [PubMed] [Google Scholar]
- 9.Dun N J, Dun S L, Hwang L L. Neurosci Lett. 1997;234:95–98. doi: 10.1016/s0304-3940(97)00666-6. [DOI] [PubMed] [Google Scholar]
- 10.Darland T, Heinricher M M, Grandy D K. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan C, Christie M J. Br J Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor M, Vaughan C, Chieng B, Christie M J. Br J Pharmacol. 1996;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughan C, Ingram S L, Christie M J. J Neurosci. 1997;17:996–1003. doi: 10.1523/JNEUROSCI.17-03-00996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meis S, Pape H C. J Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogil J S, Grisel J E, Reinscheid R K, Civelli O, Belknap J K, Grandy D K. Neurosci. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- 16.Jenck F, Moreau J-L, Martin J R, Kilpatrick G J, Reinscheid R K, Monsma F J, Jr, Nothacker H-P, Civelli O. Proc Natl Acad Sci USA. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griebel G, Perrault G, Sanger D J. Brain Res. 1999;836:221–224. doi: 10.1016/s0006-8993(99)01684-4. [DOI] [PubMed] [Google Scholar]
- 18.Kyuhou S, Gemba H. Neurosci Lett. 1999;260:113–116. doi: 10.1016/s0304-3940(98)00956-2. [DOI] [PubMed] [Google Scholar]
- 19.Vanderah T W, Raffa R B, Lashbrook J, Burritt A, Hruby V, Porreca F. Eur J Pain. 1998;2:247–280. doi: 10.1016/s1090-3801(98)90023-4. [DOI] [PubMed] [Google Scholar]
- 20.Mogil J S, Nessim L A, Wilson S G. Neurosci Lett. 1999;261:147–150. doi: 10.1016/s0304-3940(99)00012-9. [DOI] [PubMed] [Google Scholar]
- 21.Pellow S, Chopin P, File S E, Briley M. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 22.Martin J R, Bos M, Jenck F, Moreau J-L, Mutel V, Sleight A J, Wichmann J, Andrews J S, Berendsen H H G, Broekkamp C L E, et al. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- 23.Davis M, Falls W A, Campeau S, Kim M. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 24.Geller I, Seifter J. Psychopharmacologia. 1960;1:482–492. [Google Scholar]
- 25.Cook L, Davidson A B. In: Benzodiazepines. Garattini S, Mussini E, Randall L O, editors. New York: Raven; 1973. pp. 327–345. [Google Scholar]
- 26.Jenck F, Moreau J-L, Martin J R. Psychiatry Res. 1995;57:181–191. doi: 10.1016/0165-1781(95)02673-k. [DOI] [PubMed] [Google Scholar]
- 27.Moreau J-L, Scherschlicht R, Jenck F, Martin J R. Behav Pharmacol. 1995;6:682–687. [PubMed] [Google Scholar]
- 28.Martin J R, Schoch P, Jenck F, Moreau J L, Haefely W E. Psychopharmacology. 1993;111:415–422. doi: 10.1007/BF02253530. [DOI] [PubMed] [Google Scholar]
- 29.Lundstrom K, Mills A, Buell G, Allet E, Adami N, Liljeström P. Eur J Biochem. 1994;224:917–921. doi: 10.1111/j.1432-1033.1994.00917.x. [DOI] [PubMed] [Google Scholar]
- 30.Devine D P, Reinscheid R K, Monsma F J, Jr, Civelli O, Akil H. Brain Res. 1996;727:225–229. doi: 10.1016/0006-8993(96)00476-3. [DOI] [PubMed] [Google Scholar]
- 31.Walker J R, Spina M, Terenius L, Koob G F. NeuroReport. 1998;9:2243–2247. doi: 10.1097/00001756-199807130-00017. [DOI] [PubMed] [Google Scholar]
- 32.Shimohira I, Tokuyama S, Inoue M, Yamaguchi T, Takeshima H, Yoshida A, Ueda H. Soc Neurosci Abstr. 1998;24:1356. [Google Scholar]
- 33.Mathis J P, Ryan-Moro J, Chang A, Hom J S H, Scheinberg D A, Pasternak G W. Biochem Biophys Res Commun. 1997;230:462–465. doi: 10.1006/bbrc.1996.5867. [DOI] [PubMed] [Google Scholar]
- 34.Pan Y X, Xu J, Wan B L, Zuckerman A, Pasternak G W. FEBS Lett. 1998;435:65–68. doi: 10.1016/s0014-5793(98)01039-4. [DOI] [PubMed] [Google Scholar]