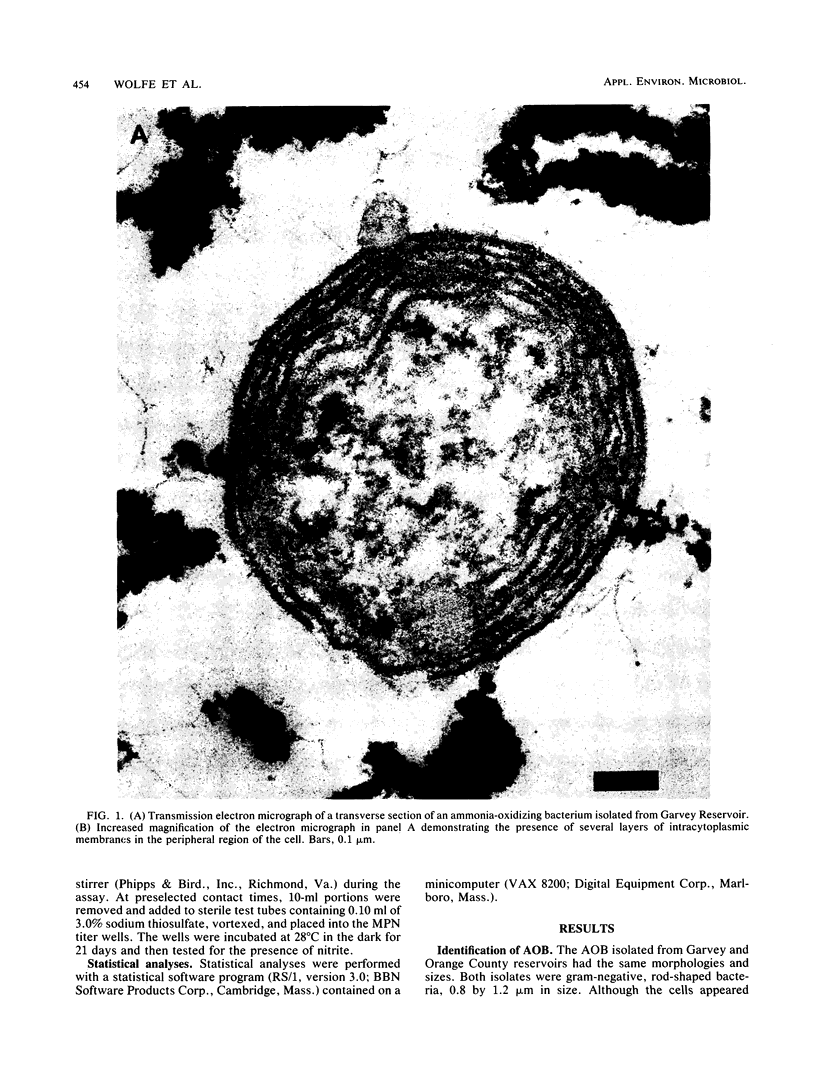
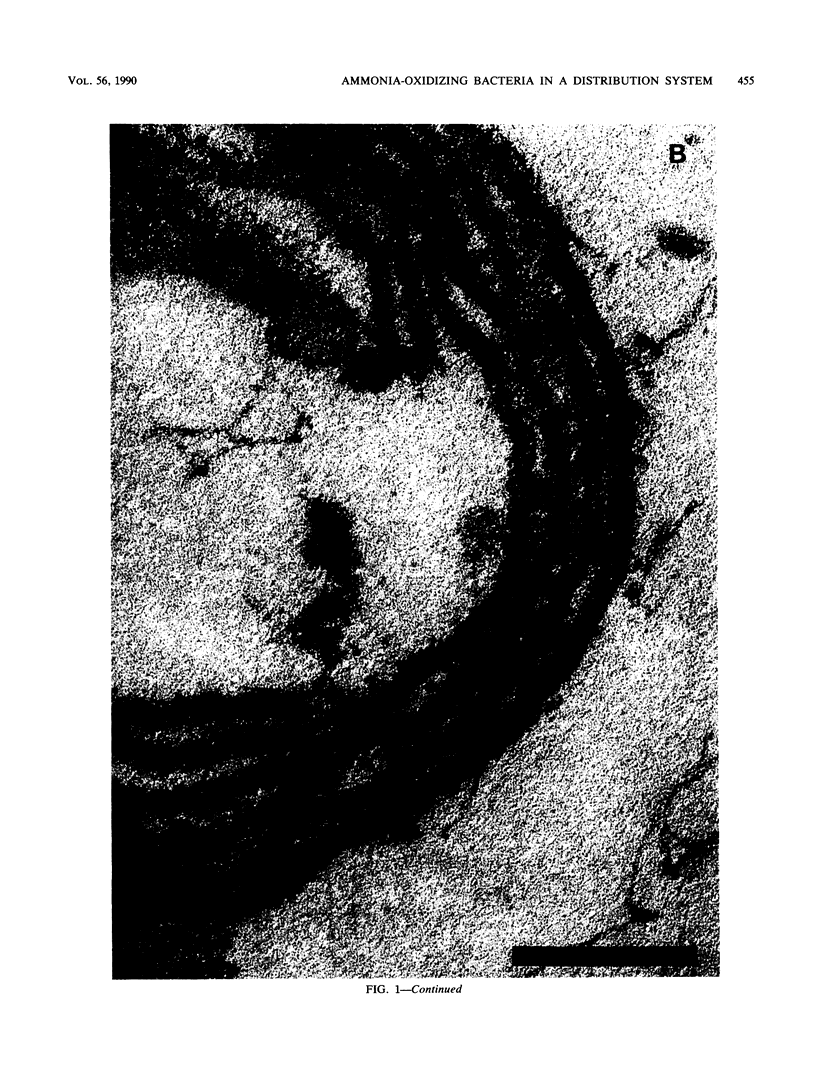
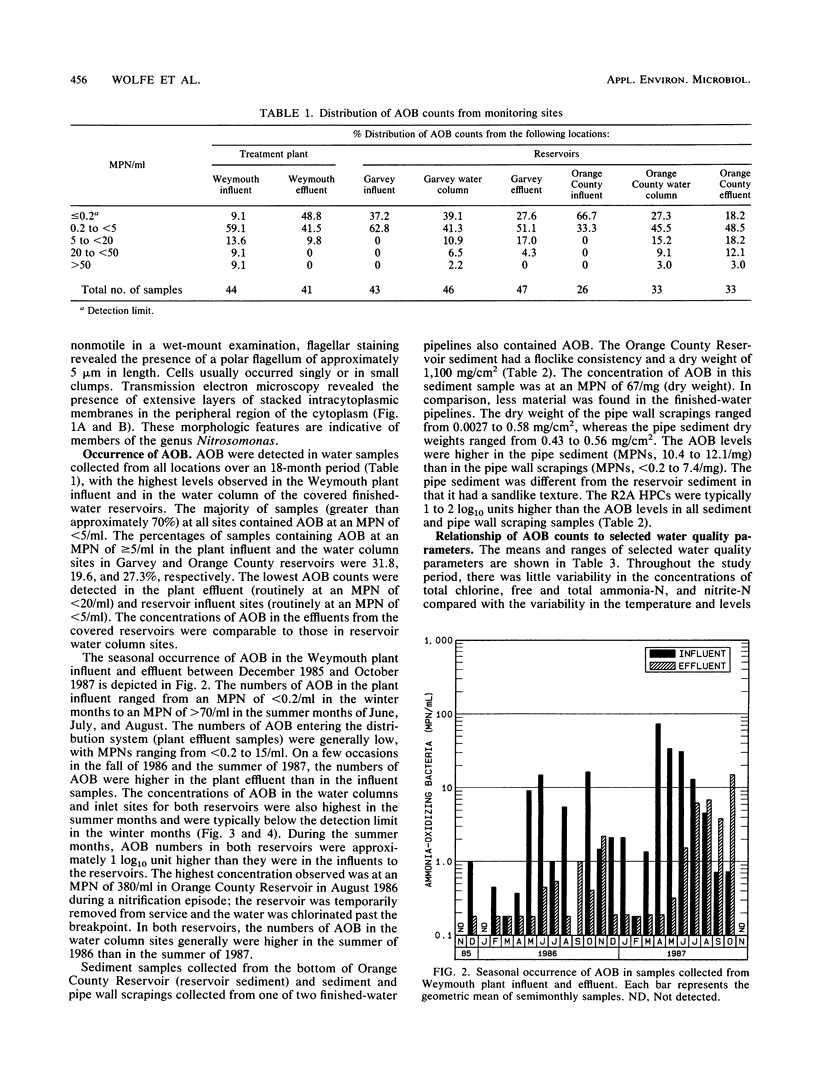
Abstract
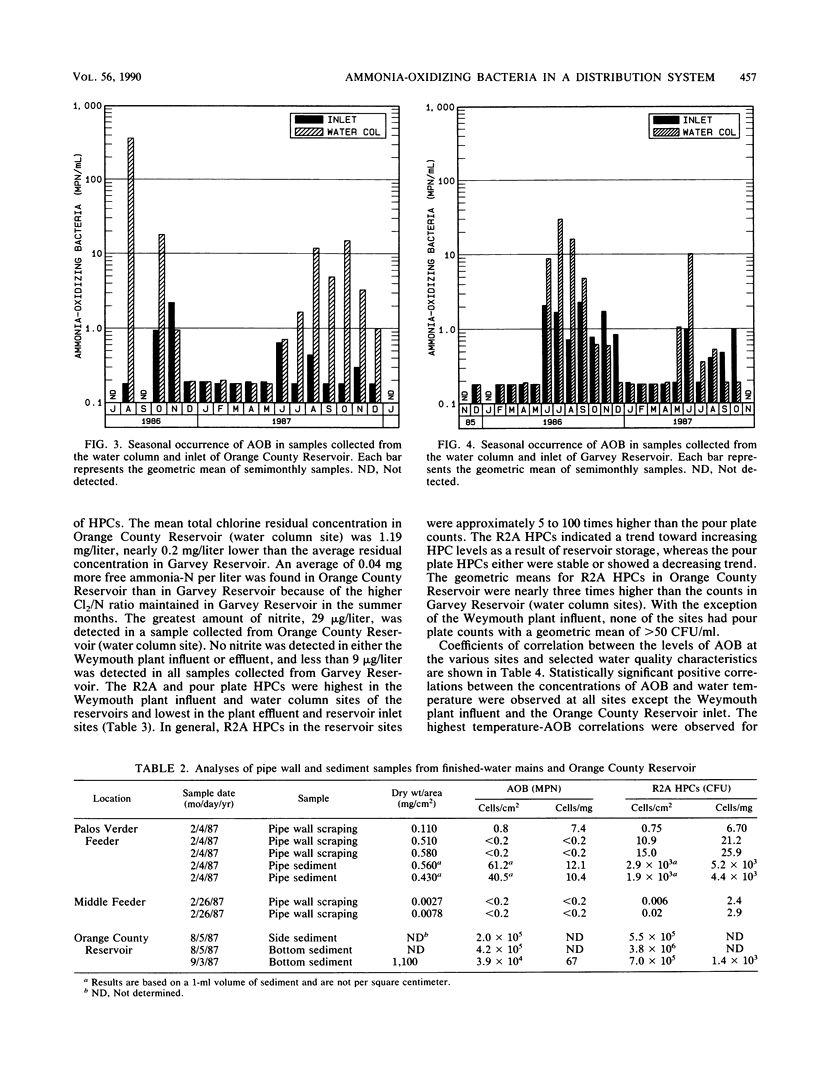
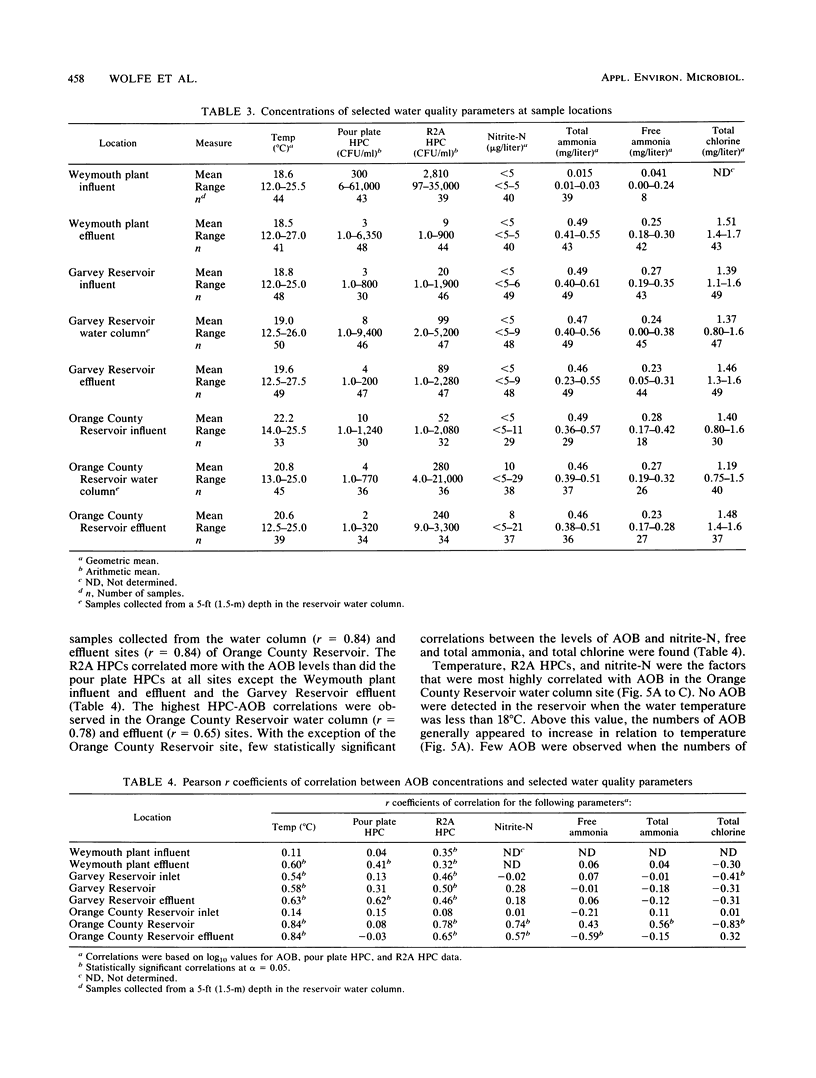
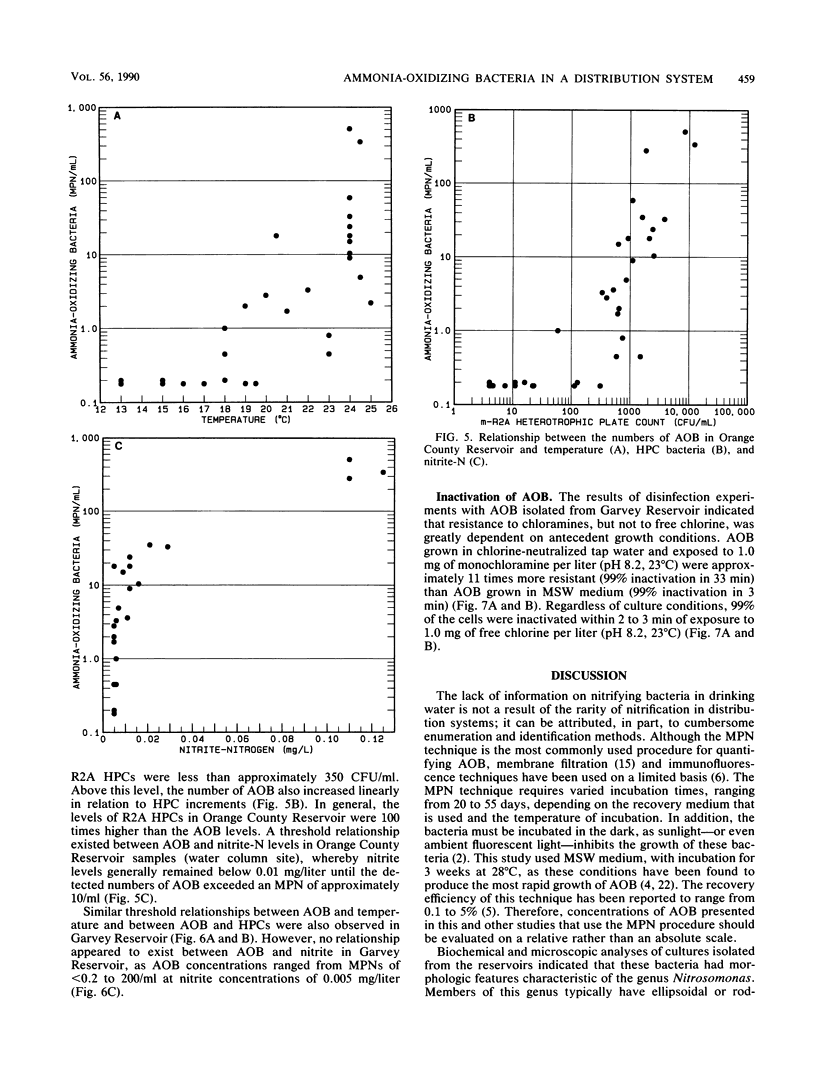
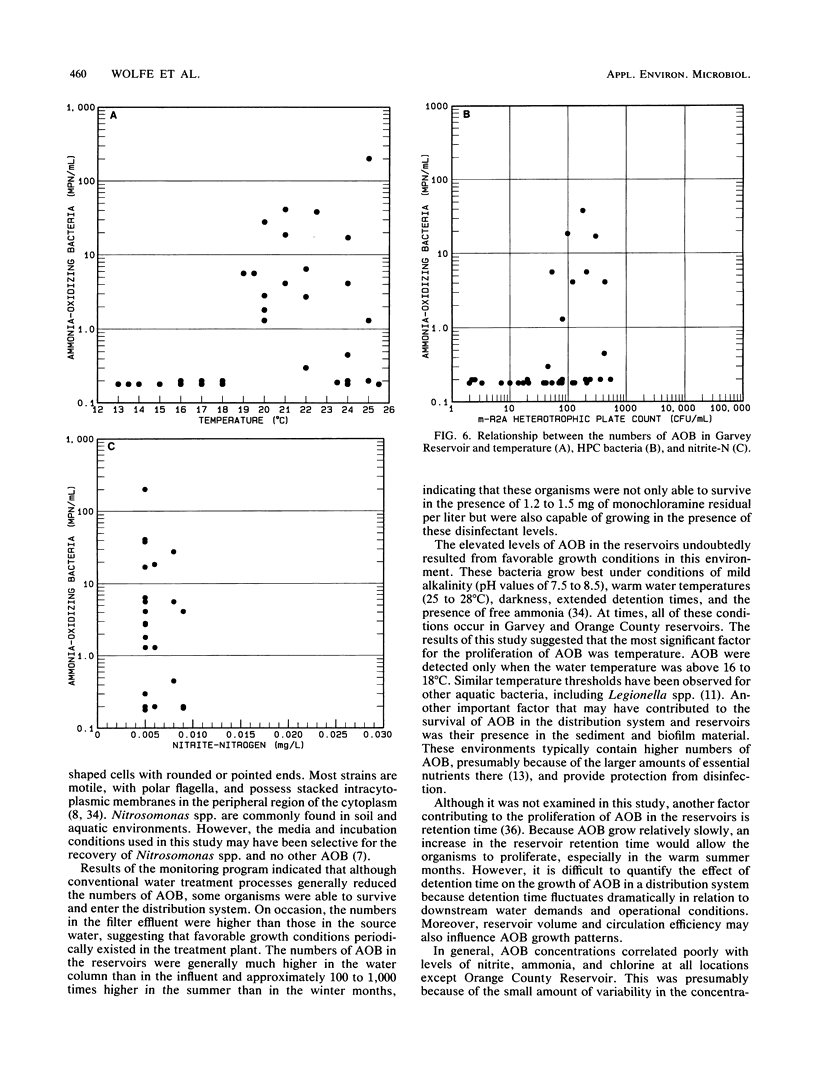
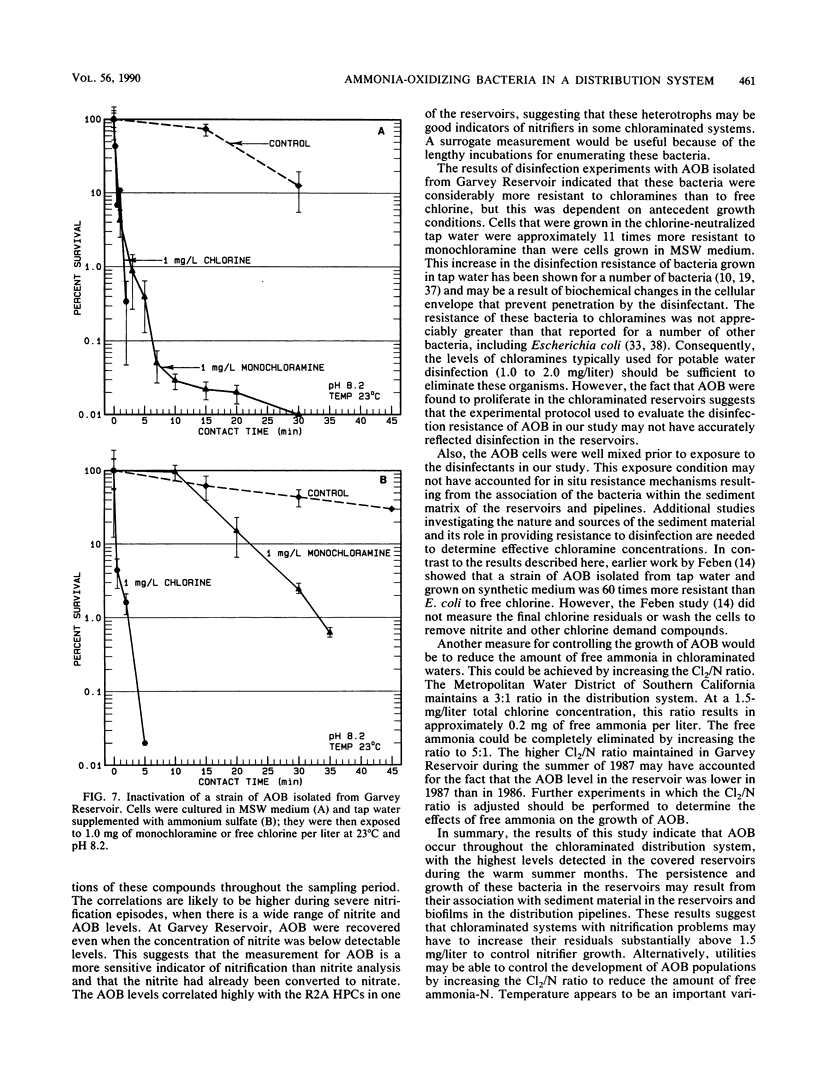
Nitrification in chloraminated drinking water can have a number of adverse effects on water quality, including a loss of total chlorine and ammonia-N and an increase in the concentration of heterotrophic plate count bacteria and nitrite. To understand how nitrification develops, a study was conducted to examine the factors that influence the occurrence of ammonia-oxidizing bacteria (AOB) in a chloraminated distribution system. Samples were collected over an 18-month period from a raw-water source, a conventional treatment plant effluent, and two covered, finished-water reservoirs that previously experienced nitrification episodes. Sediment and biofilm samples were collected from the interior wall surfaces of two finished-water pipelines and one of the covered reservoirs. The AOB were enumerated by a most-probable-number technique, and isolates were isolated and identified. The resistance of naturally occurring AOB to chloramines and free chlorine was also examined. The results of the monitoring program indicated that the levels of AOB, identified as members of the genus Nitrosomonas, were seasonally dependent in both source and finished waters, with the highest levels observed in the warm summer months. The concentrations of AOB in the two reservoirs, both of which have floating covers made of synthetic rubber (Hypalon; E.I. du Pont de Nemours & Co., Inc., Wilmington, Del.), had most probable numbers that ranged from less than 0.2 to greater than 300/ml and correlated significantly with temperature and levels of heterotrophic plate count bacteria. No AOB were detected in the chloraminated reservoirs when the water temperature was below 16 to 18 degrees C. The study indicated that nitrifiers occur throughout the chloraminated distribution system. Higher concentrations of AOB were found in the reservoir and pipe sediment materials than in the pipe biofilm samples. The AOB were approximately 13 times more resistant to monochloramine than to free chlorine. After 33 min of exposure to 1.0 mg of monochloramine per liter (pH 8.2, 23 degrees C), 99% of an AOB culture was inactivated. The amounts of this disinfectant that are currently used (1.5 mg/liter at a 3:1 ratio of chlorine to ammonia-N) may be inadequate to control the growth of these organisms in the distribution system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser L. W., Mays E. L. Use of nitrifier activity measurements to estimate the efficiency of viable nitrifier counts in soils and sediments. Appl Environ Microbiol. 1982 Apr;43(4):945–948. doi: 10.1128/aem.43.4.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W. Population ecology of nitrifying bacteria. Annu Rev Microbiol. 1979;33:309–333. doi: 10.1146/annurev.mi.33.100179.001521. [DOI] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Diversity in the ammonia-oxidizing nitrifier population of a soil. Appl Environ Microbiol. 1978 Oct;36(4):584–588. doi: 10.1128/aem.36.4.584-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Serological diversity within a terrestrial ammonia-oxidizing population. Appl Environ Microbiol. 1978 Oct;36(4):589–593. doi: 10.1128/aem.36.4.589-593.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L. A., Favero M. S., Bond W. W., Petersen N. J. Factors affecting comparative resistance of naturally occurring and subcultured Pseudomonas aeruginosa to disinfectants. Appl Microbiol. 1972 May;23(5):863–869. doi: 10.1128/am.23.5.863-869.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne J. S., Trew R. M. Presence of Legionella in London's water supplies. Isr J Med Sci. 1986 Sep;22(9):633–639. [PubMed] [Google Scholar]
- Kuchta J. M., States S. J., McGlaughlin J. E., Overmeyer J. H., Wadowsky R. M., McNamara A. M., Wolford R. S., Yee R. B. Enhanced chlorine resistance of tap water-adapted Legionella pneumophila as compared with agar medium-passaged strains. Appl Environ Microbiol. 1985 Jul;50(1):21–26. doi: 10.1128/aem.50.1.21-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulewich V. A., Strom P. F., Finstein M. S. Length of incubation for enumerating nitrifying bacteria present in various environments. Appl Microbiol. 1975 Feb;29(2):265–268. doi: 10.1128/am.29.2.265-268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S., Walker N. Isolation of ammonia-oxidizing autotrophic bacteria. J Appl Bacteriol. 1968 Dec;31(4):493–497. doi: 10.1111/j.1365-2672.1968.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Ward N. R., Wolfe R. L., Olson B. H. Effect of pH, application technique, and chlorine-to-nitrogen ratio on disinfectant activity of inorganic chloramines with pure culture bacteria. Appl Environ Microbiol. 1984 Sep;48(3):508–514. doi: 10.1128/aem.48.3.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]