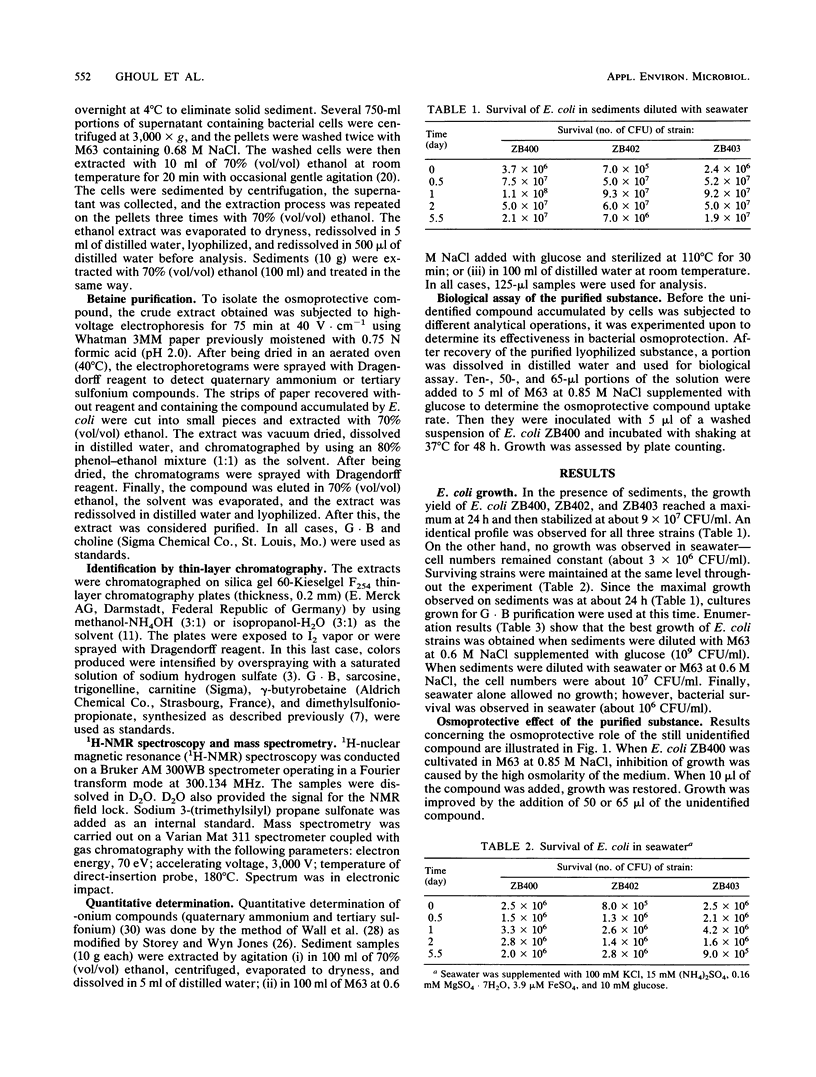
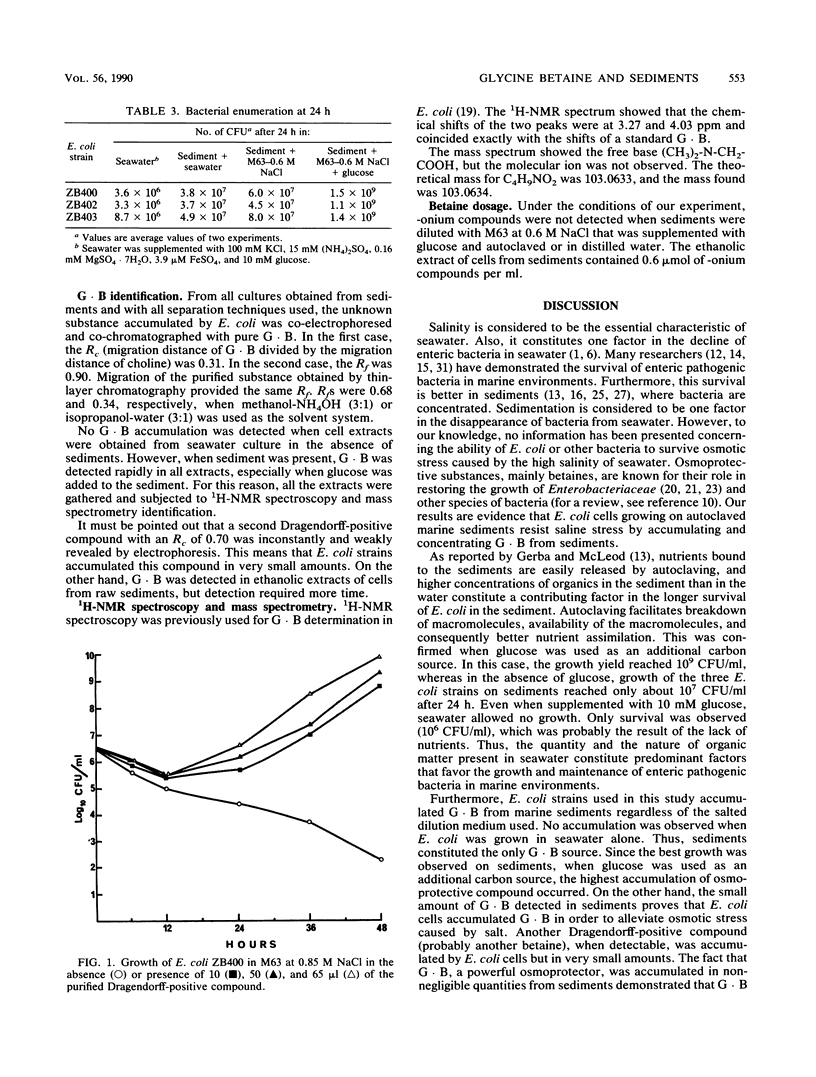
Abstract
Escherichia coli grew faster in autoclaved marine sediment than in seawater alone. When E. coli was cultivated in sediment diluted with minimal medium M63 at 0.6 M NaCl, supplemented or not supplemented with glucose or with seawater, the osmoprotector glycine betaine was accumulated in the cells. The best growth occurred on glucose. Accumulation of glycine betaine was not observed with E. coli was grown in sterile seawater alone. The fact that E. coli grew better in the sediments than in seawater is attributed somewhat to the high content of organic matter in the sediment but mainly to the accumulation of glycine betaine. Thus, osmoprotection should be considered to be an additional factor in bacterial survival in estuarine sediments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M., Kator H. Sublethal stress in Escherichia coli: a function of salinity. Appl Environ Microbiol. 1979 Dec;38(6):1147–1152. doi: 10.1128/aem.38.6.1147-1152.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers J. R. The species distribution of some naturally-occurring quaternary ammonium compounds. Comp Biochem Physiol. 1967 Apr;21(1):11–21. doi: 10.1016/0010-406x(67)90109-0. [DOI] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. An evaluation of factors affecting the survival of Escherichia coli in sea water. II. Salinity, pH, and nutrients. Appl Microbiol. 1960 Jul;8:247–250. doi: 10.1128/am.8.4.247-250.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. Factors affecting the survival of bacteria in sea water. Appl Microbiol. 1959 Nov;7:388–392. doi: 10.1128/am.7.6.388-392.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Chambers S. T., Kunin C. M., Miller D., Hamada A. Dimethylthetin can substitute for glycine betaine as an osmoprotectant molecule for Escherichia coli. J Bacteriol. 1987 Oct;169(10):4845–4847. doi: 10.1128/jb.169.10.4845-4847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., McLeod J. S. Effect of sediments on the survival of Escherichia coli in marine waters. Appl Environ Microbiol. 1976 Jul;32(1):114–120. doi: 10.1128/aem.32.1.114-120.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. J., Atwell R. W., Brayton P. R., Palmer L. M., Rollins D. M., Roszak D. B., Singleton F. L., Tamplin M. L., Colwell R. R. The fate of enteric pathogenic bacteria in estuarine and marine environments. Microbiol Sci. 1986 Nov;3(11):324–329. [PubMed] [Google Scholar]
- King Gary M. Methanogenesis from Methylated Amines in a Hypersaline Algal Mat. Appl Environ Microbiol. 1988 Jan;54(1):130–136. doi: 10.1128/aem.54.1.130-136.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Shiaris M. P., Rex A. C., Pettibone G. W., Keay K., McManus P., Rex M. A., Ebersole J., Gallagher E. Distribution of indicator bacteria and Vibrio parahaemolyticus in sewage-polluted intertidal sediments. Appl Environ Microbiol. 1987 Aug;53(8):1756–1761. doi: 10.1128/aem.53.8.1756-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



