Abstract
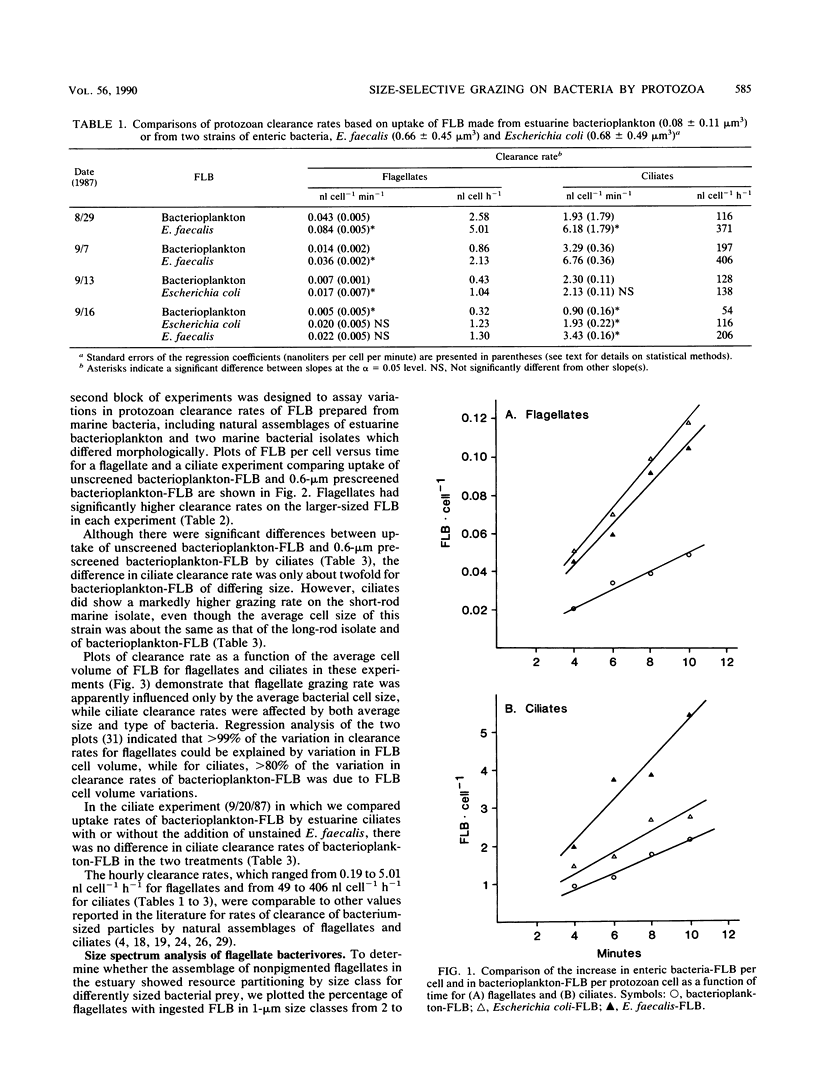
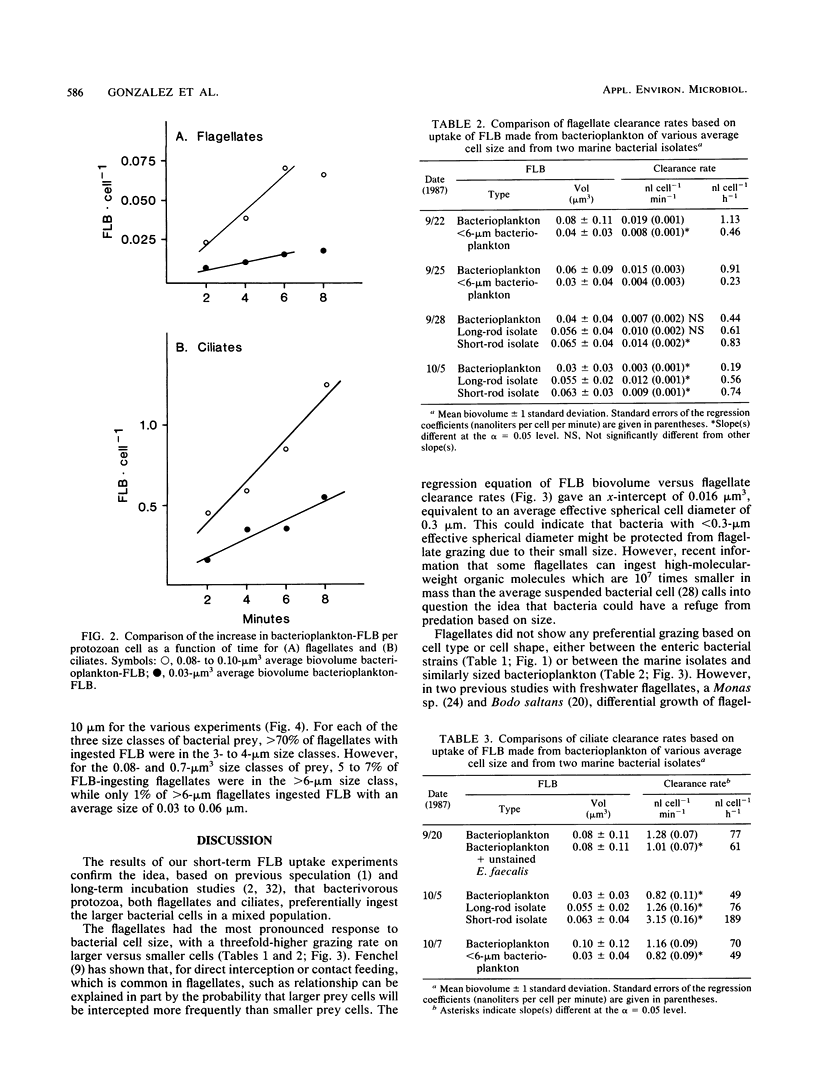
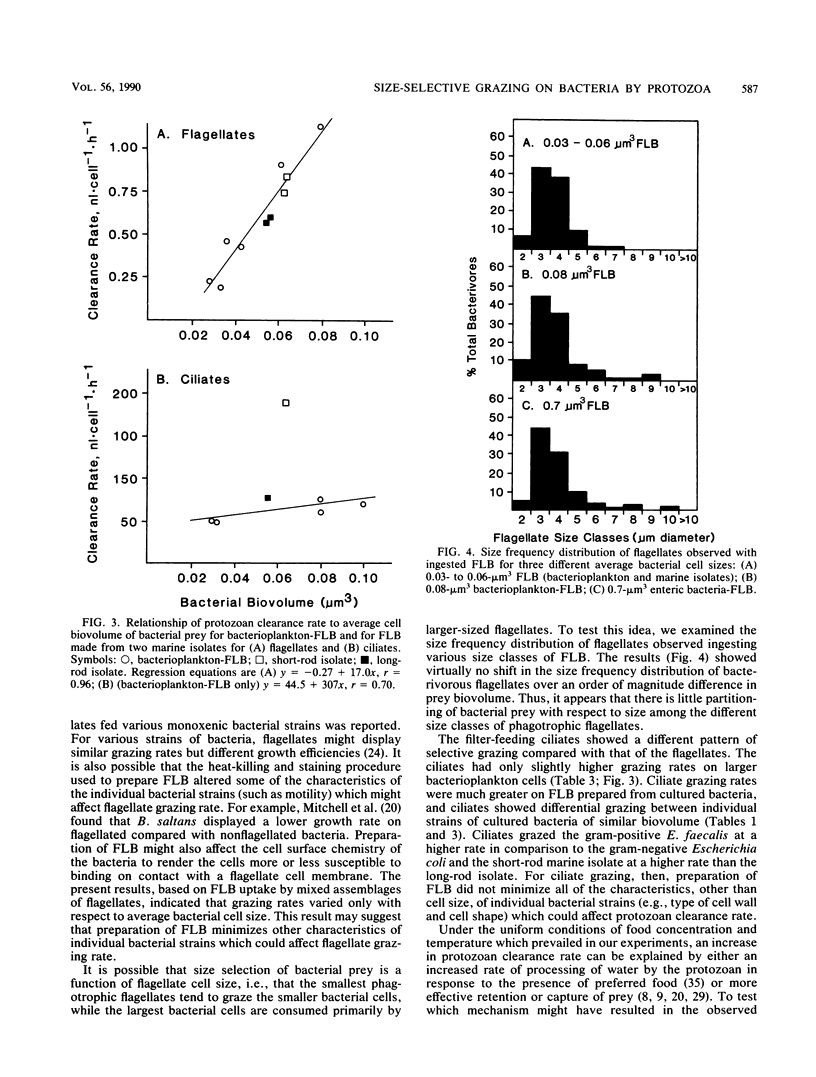
The small average cell size of in situ bacterioplankton, relative to cultured cells, has been suggested to be at least partly a result of selection of larger-sized cells by bacterivorous protozoa. In this study, we determined the relative rates of uptake of fluorescence-labeled bacteria (FLB), of various cell sizes and cell types, by natural assemblages of flagellates and ciliates in estuarine water. Calculated clearance rates of bacterivorous flagellates had a highly significant, positive relationship with size of FLB, over a range of average biovolume of FLB of 0.03 to 0.08 microns3. Bacterial cell type or cell shape per se did not appear to affect flagellate clearance rates. The dominant size classes of flagellates which ingested all types of FLB were 3- to 4-microns cells. Ciliates also showed a general preference for larger-sized bacteria. However, ciliates ingested a gram-positive enteric bacterium and a marine bacterial isolate at higher rates than they did a similarly sized, gram-negative enteric bacterium or natural bacterioplankton, respectively. From the results of an experiment designed to test whether the addition of a preferentially grazed bacterial strain stimulated clearance rates of natural bacterioplankton FLB by the ciliates, we hypothesized that measured differences in rates of FLB uptake were due instead to differences in effective retention of bacteria by the ciliates. In general, clearance rates for different FLB varied by a factor of 2 to 4. Selective grazing by protozoa of larger bacterioplankton cells, which are generally the cells actively growing or dividing, may in part explain the small average cell size, low frequency of dividing cells, and low growth rates generally observed for assemblages of suspended bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloem J., Starink M., Bär-Gilissen M. J., Cappenberg T. E. Protozoan grazing, bacterial activity, and mineralization in two-stage continuous cultures. Appl Environ Microbiol. 1988 Dec;54(12):3113–3121. doi: 10.1128/aem.54.12.3113-3121.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Stoecker D. K. Effects of fixation on cell volume of marine planktonic protozoa. Appl Environ Microbiol. 1989 Jul;55(7):1761–1765. doi: 10.1128/aem.55.7.1761-1765.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Fuhrman J. A. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton. Appl Environ Microbiol. 1987 Jun;53(6):1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T. Carbon and nitrogen content of natural planktonic bacteria. Appl Environ Microbiol. 1986 Jul;52(1):28–32. doi: 10.1128/aem.52.1.28-32.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Berman T. Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol. 1983 Apr;45(4):1196–1201. doi: 10.1128/aem.45.4.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Rassoulzadegan F. Rates of digestion of bacteria by marine phagotrophic protozoa: temperature dependence. Appl Environ Microbiol. 1988 May;54(5):1091–1095. doi: 10.1128/aem.54.5.1091-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



