Abstract
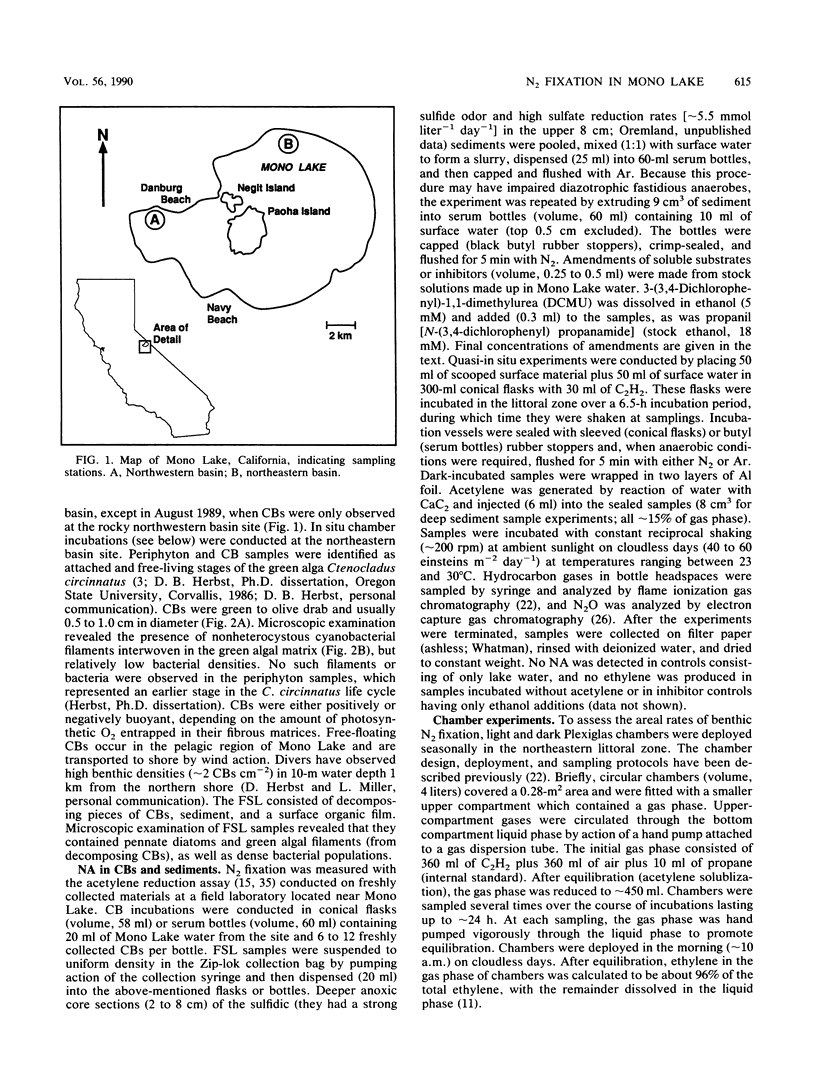
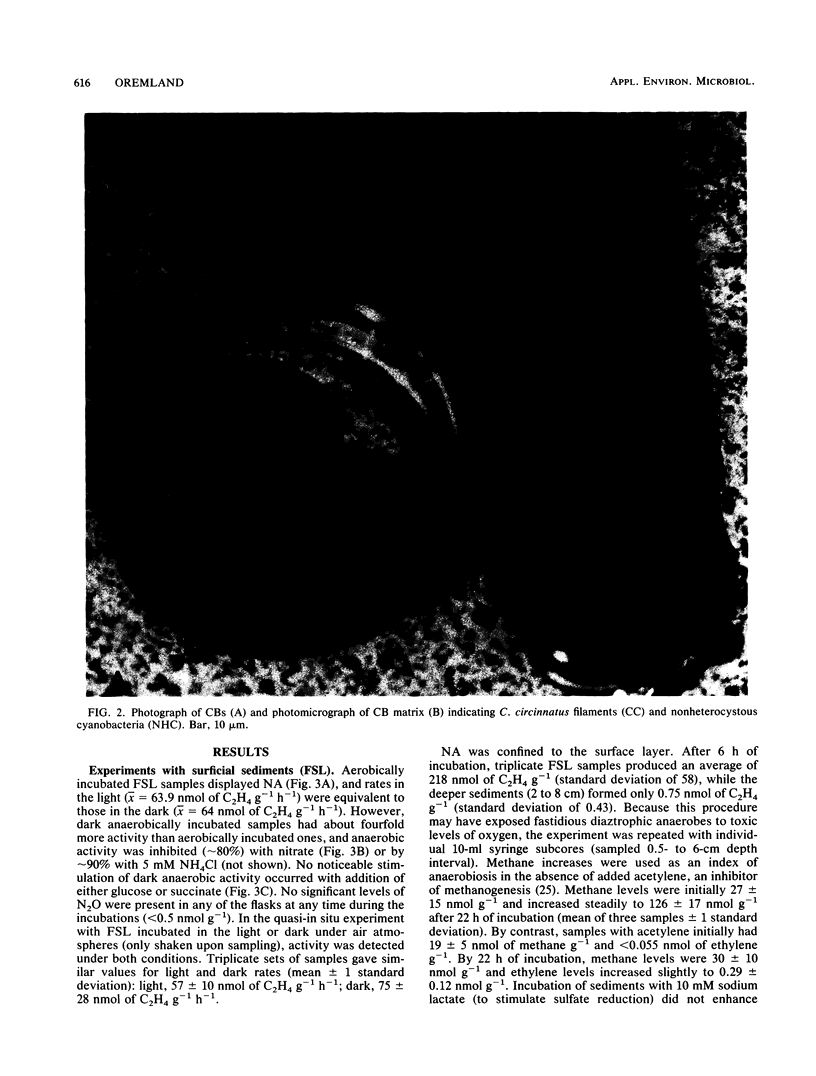
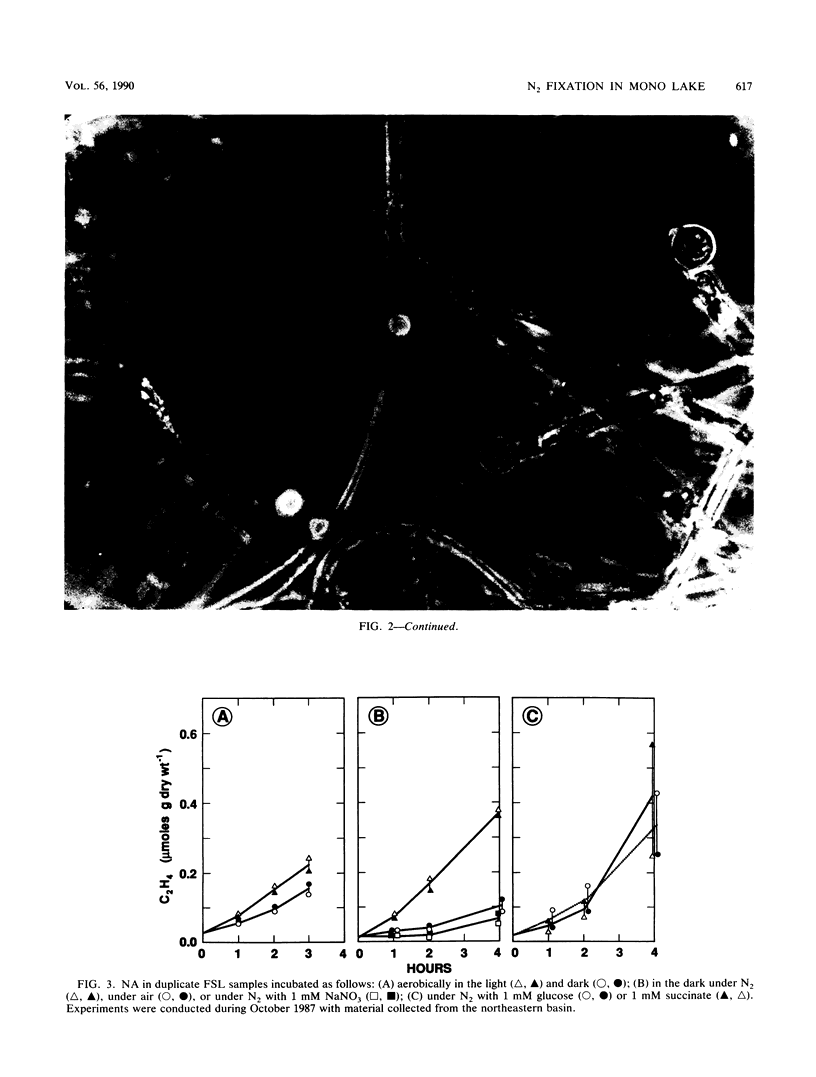
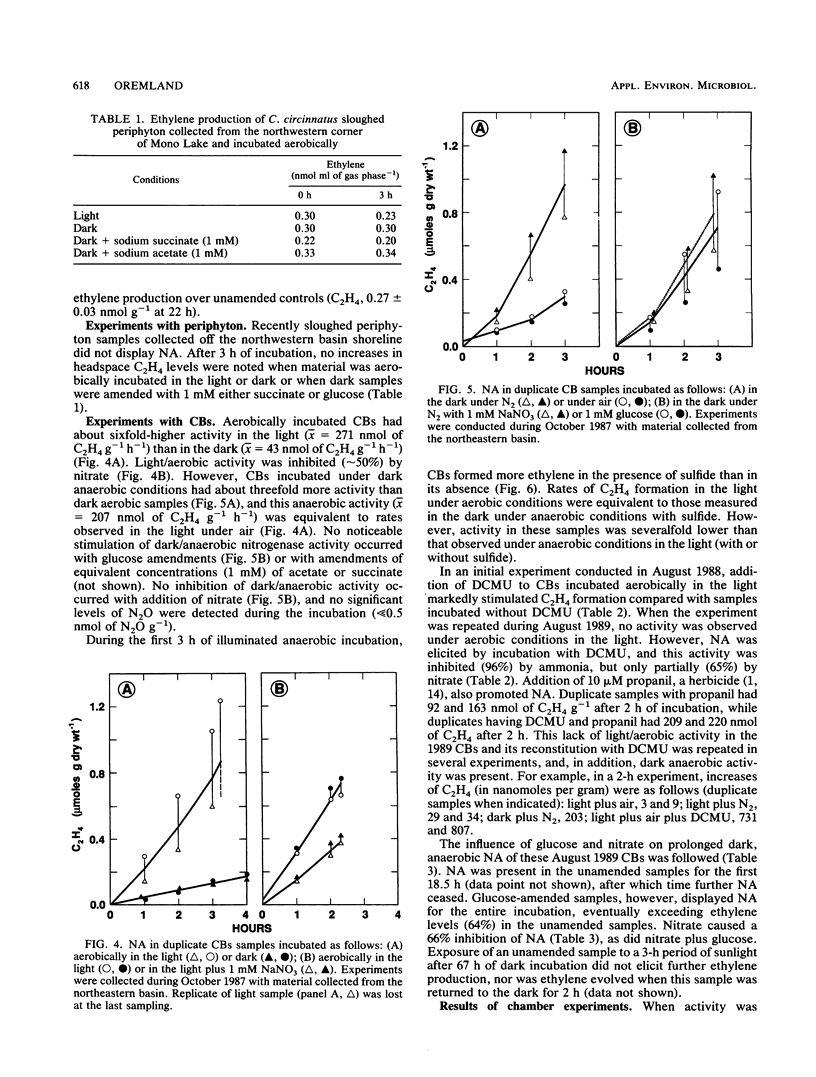
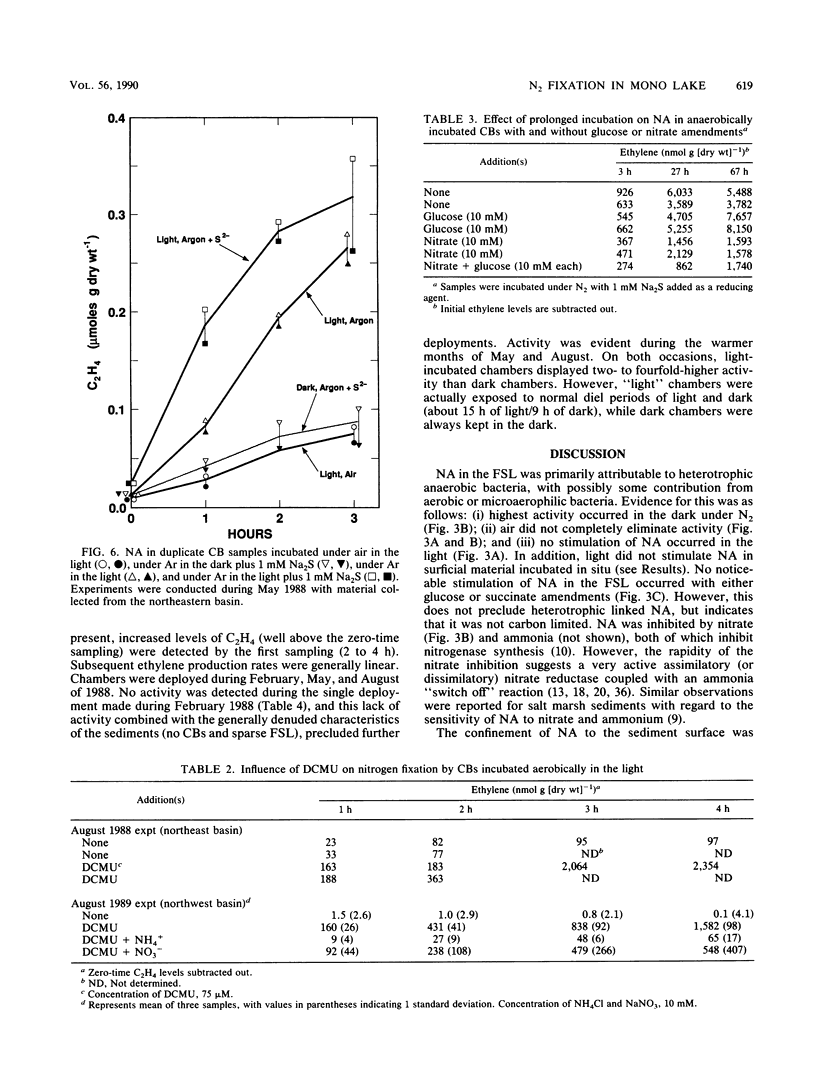
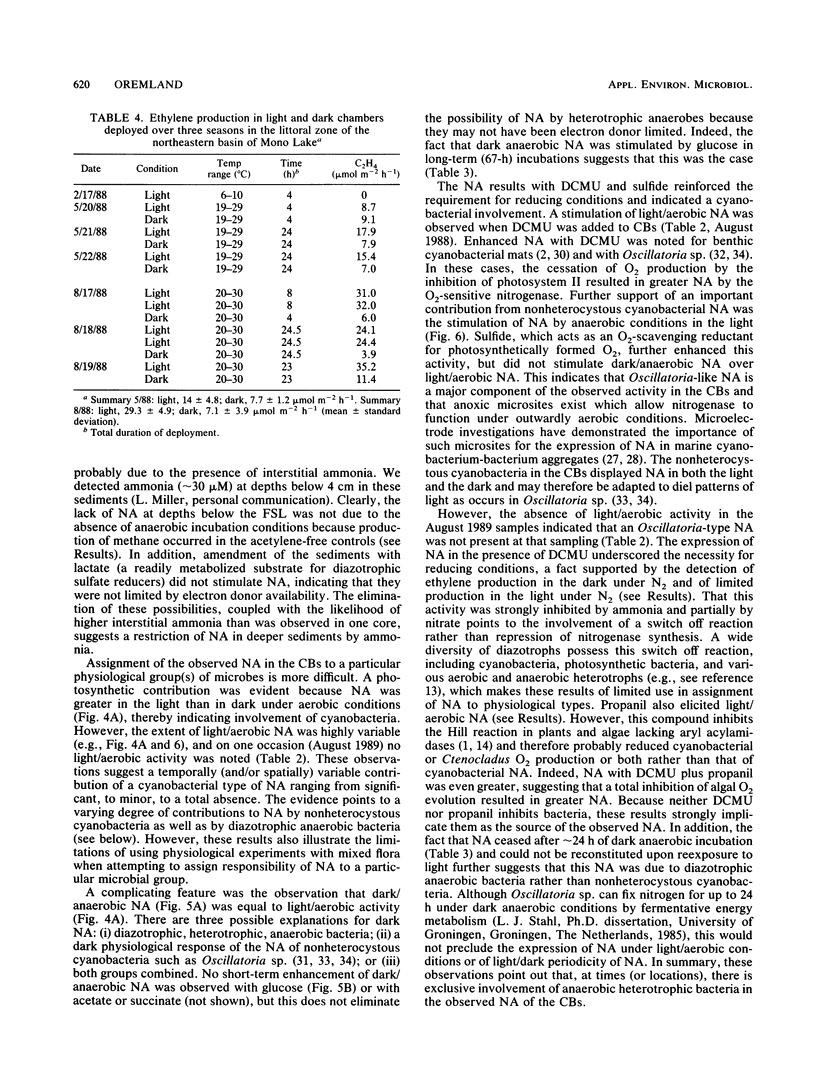
Two types of diazotrophic microbial communities were found in the littoral zone of alkaline hypersaline Mono Lake, California. One consisted of anaerobic bacteria inhabiting the flocculent surface layers of sediments. Nitrogen fixation (acetylene reduction) by flocculent surface layers occurred under anaerobic conditions, was not stimulated by light or by additions of organic substrates, and was inhibited by O2, nitrate, and ammonia. The second community consisted of a ball-shaped association of a filamentous chlorophyte (Ctenocladus circinnatus) with diazotrophic, nonheterocystous cyanobacteria, as well as anaerobic bacteria (Ctenocladus balls). Nitrogen fixation by Ctenocladus balls was usually, but not always, stimulated by light. Rates of anaerobic dark fixation equaled those in the light under air. Fixation in the light was stimulated by 3-(3,4-dichlorophenyl)-1, 1-dimethylurea and by propanil [N-(3,4-dichlorophenyl)propanamide]. 3-(3,4-Dichlorophenyl)-1,1-dimethyl urea-elicited nitrogenase activity was inhibited by ammonia (96%) and nitrate (65%). Fixation was greatest when Ctenocladus balls were incubated anaerobically in the light with sulfide. Dark anaerobic fixation was not stimulated by organic substrates in short-term (4-h) incubations, but was in long-term (67-h) ones. Areal estimates of benthic N2 fixation were measured seasonally, using chambers. Highest rates (∼29.3 μmol of C2H4 m−2 h−1) occurred under normal diel regimens of light and dark. These estimates indicate that benthic N2 fixation has the potential to be a significant nitrogen source in Mono Lake.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bebout B. M., Paerl H. W., Crocker K. M., Prufert L. E. Diel interactions of oxygenic photosynthesis and n(2) fixation (acetylene reduction) in a marine microbial mat community. Appl Environ Microbiol. 1987 Oct;53(10):2353–2362. doi: 10.1128/aem.53.10.2353-2362.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone D. G., Carpenter E. J. Nitrogen fixation in the marine environment. Science. 1982 Sep 17;217(4565):1140–1142. doi: 10.1126/science.217.4565.1140. [DOI] [PubMed] [Google Scholar]
- Drozd J. W., Tubb R. S., Postgate J. R. A chemostat study of the effect of fixed nitrogen sources on nitrogen fixation, membranes and free amino acids in Azotobacter chroococcum. J Gen Microbiol. 1972 Nov;73(2):221–232. doi: 10.1099/00221287-73-2-221. [DOI] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Fu H., Burris R. H. Ammonium inhibition of nitrogenase activity in Herbaspirillum seropedicae. J Bacteriol. 1989 Jun;171(6):3168–3175. doi: 10.1128/jb.171.6.3168-3175.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. K., Shah V. K., Brill W. J. Feedback inhibition of nitrogenase. J Bacteriol. 1981 Dec;148(3):884–888. doi: 10.1128/jb.148.3.884-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habte M., Alexander M. Nitrogen fixation by photosynthetic bacteria in lowland rice culture. Appl Environ Microbiol. 1980 Feb;39(2):342–347. doi: 10.1128/aem.39.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. S., Hales B. J., Socolofsky M. D. Nitrogen fixation and ammonia switch-off in the photosynthetic bacterium Rhodopseudomonas viridis. J Bacteriol. 1983 Jul;155(1):107–112. doi: 10.1128/jb.155.1.107-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugkist J., Haaker H. Inhibition of nitrogenase activity by ammonium chloride in Azotobacter vinelandii. J Bacteriol. 1984 Jan;157(1):148–151. doi: 10.1128/jb.157.1.148-151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Taylor B. F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975 Oct;30(4):707–709. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Prufert L. E. Oxygen-poor microzones as potential sites of microbial n(2) fixation in nitrogen-depleted aerobic marine waters. Appl Environ Microbiol. 1987 May;53(5):1078–1087. doi: 10.1128/aem.53.5.1078-1087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triska F. J., Oremland R. S. Denitrification associated with periphyton communities. Appl Environ Microbiol. 1981 Oct;42(4):745–748. doi: 10.1128/aem.42.4.745-748.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]




