Abstract
We report unprecedented numbers of juvenile snake pipefish, Entelurus aequoreus, in continuous plankton records of the Northeastern Atlantic since 2002. Increased sea surface temperatures (SSTs) in the Northern Hemisphere, linked to global warming, are a likely cause. Analysis of a long-term time-series of SST data in the Northeastern Atlantic shows a rise in winter, spring and summer sea temperatures (January–September), when the eggs of E. aqueoreus, which are brooded by the male, are developing and the larvae are growing in plankton. From what is known of the reproductive biology of closely related species, we suggest that the increased abundance of larval and juvenile E. aequoreus in the plankton as far west as the Mid-Atlantic Ridge may reflect the impact of temperature on abundance, through its effects on the operational sex ratio and potential reproductive rate, the onset of the breeding season and juvenile survival in this sex role reversed fish.
Keywords: climate, Entelurus, plankton, operational sex ratio, sex role reversal
1. Introduction
Climate-induced increases in sea surface temperature (SST) have occurred in the Northeastern Atlantic in recent years (Hurrell & van Loon 1997) that have altered the distributions of many fish species (Quero et al. 1998; Brander et al. 2003). Through its impact on physiological processes, increased sea temperature is also likely to affect many other aspects of the biology of fishes that may also affect their abundance, such as reproduction, larval survival and recruitment. For example, within the thermal niche limits of a species, warmer temperatures may advance the start of the breeding season (Winters & Wheeler 1996) and, through the influence of temperature upon growth rate, shorten the duration of the juvenile stage (Hakala et al. 2003; Martell et al. 2005), which is a period of high mortality (Leggett & Deblois 1994), thus improving recruitment (Cushing & Horwood 1994).
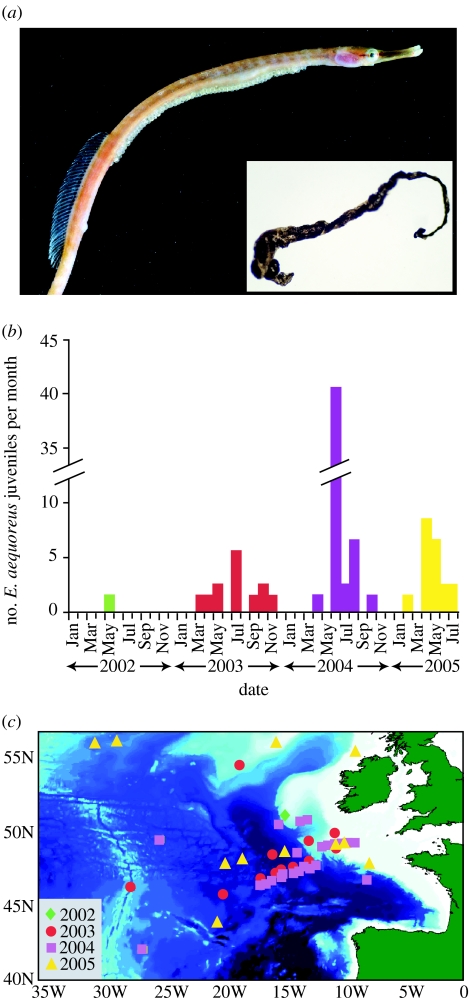
Apart from changes in the distribution of fishes, climate-induced changes have occurred in the communities of planktonic organisms in the North Atlantic (Beaugrand et al. 2002). Many of these changes have been detected using samples collected by the continuous plankton recorder (CPR) survey (Batten et al. 2003), which has sampled the North Atlantic on a monthly basis since 1931. Here, we report unprecedented numbers of juvenile snake pipefishes, Entelurus aequoreus (figure 1a), in CPR samples from the Northeastern Atlantic since 2002.
Figure 1.
Abundance and spatial distribution of snake pipefish in CPR samples from the Northeastern Atlantic. (a) A brooding adult male E. aequoreus (male length up to 40 cm; female length up to 60 cm; image, David Shale, Ph.D.) with eggs clearly visible on the abdomen and, inset, a juvenile E. aequoreus sampled by the CPR in 2005 (length 1 cm). The identity of the juvenile pipefishes was confirmed by genetic analysis (GenBank accession number DQ437522). (b) The total monthly abundance of planktonic larval and juvenile E. aequoreus in Northeastern Atlantic CPR samples from 2002 to 2005 in the areas shown in c. (c) The distribution of planktonic larval and juvenile E. aequoreus in CPR samples in the Northeastern Atlantic from 2002 to 2005.
Like other Syngnathidae (seahorses and pipefishes), male E. aequoreus brood the eggs (Wilson et al. 2003), which the female lays on a specialised incubation area on the males abdomen (figure 1a). In the deep snouted pipefish, Sygnathus typhle, males are unavailable to mate when brooding, and thus it is the males' brooding rate that limits the female potential reproductive rate (PRR; Ahnesjö 1995). This difference in reproductive rates between the sexes creates a female-biased operational sex ratio (OSR) and reverses the sex roles, because females now compete among each other for access to mates (Kvarnemo & Ahnesjö 2002). We suggest that the increased abundance of young E. aequoreus (larvae and juveniles) in CPR samples may reflect the impact of warmer sea temperatures on reproduction and larval survival in this species, and we speculate on the influence of climate change on sex role dynamics.
2. Material and methods
(a) Plankton and sea surface temperature data
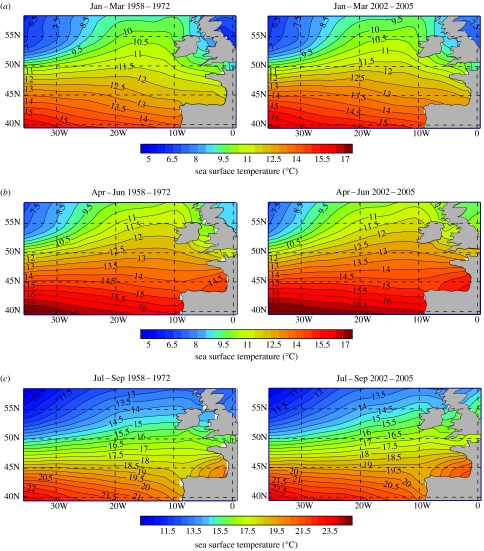
Northeastern Atlantic plankton samples have been collected over a 75-year period by the CPR, an upper layer plankton sampler towed behind merchant ships on regular routes. Seawater enters the CPR through a front aperture and the plankton are retained on a moving band of silk gauze that is slowly wound into a tank of formalin (Batten et al. 2003). In the laboratory, the gauze is cut into sections (a CPR sample), each representing the plankton from 3 m3 of water taken during 10 nautical miles (18 km) of tow, and up to 450 taxa are identified and enumerated. Average SST data for the Northeastern Atlantic from January to September inclusive (figure 2) were obtained from the HadISST dataset (Met Office, Hadley Centre for Climate Change and Research) for the intervals 1958–1972 and 2002–2005 inclusive; these represent the periods of published data on pipefish occurrence in CPR samples (Coombs 1980; this study).
Figure 2.
Comparison of Northeastern Atlantic winter (January to March), spring (April to June) and summer (July to September) average SST (°C) for the two periods of published data on larval and juvenile pipefish occurrence in CPR samples (Coombs 1980; this study). (a) Winter, (b) spring and (c) summer (note different scale).
(b) Identification of larval and juvenile pipefishes in CPR samples
The identities of pipefish larvae and juveniles in CPR samples collected in 2005 from the Northeastern Atlantic were confirmed by genetic analysis and comparison to GenBank. Small tissue samples (approx. 1 mm length) from individual specimens were placed separately into 180 μl of chelex solution (Instagene Matrix, Biorad) together with 6 μl of 1 M DTT, 4 μl of proteinase-K (10 mg ml−1) and 10 μl of 10% SDS and incubated at 55°C for 4 h. Each sample was then vortexed briefly and centrifuged at 12 000g for 15 s, heated at 105°C for 10 min in a dry-block heater, vortexed for 10 s and centrifuged at 12 000g for 3 min. The supernatant was transferred to a Micropure-EZ centrifugal filter device (CFD; Millipore Corp.) that was inserted into a Microcon YM-30 CFD (Millipore Corp.) and the combined unit was centrifuged at 14 000g for 8 min. After discarding the Micropure-EZ CFD, the sample retained in the YM-30 was washed three times with 200 μl of sterile water; the first two washes were centrifuged at 14 000g for 8 min and the final wash was centrifuged at 14 000g for 5 min. The retained DNA was then recovered. All centrifugation steps were performed at 22°C. A partial mtDNA 16S rDNA gene sequence was amplified from 1 μl of DNA template by PCR using the mtDNA 16S rDNA gene primers 16sar-L and 16sbr-H (Palumbi et al. 1991), and sequenced.
3. Results
Monthly plankton samples collected over the past 57 years in the Northeastern Atlantic by the CPR survey reveal an increase in abundance and extended presence in the plankton of juvenile pipefishes. Morphological and genetic analyses indicated that these were E. aequoreus juveniles (figure 1a). From 1958 to 2002, juvenile pipefishes occurred only occasionally in CPR samples (published data until 1972 (Coombs 1980), thereafter personal observations). Since 2002, they appear in CPR samples from February to November (figure 1b). For example, more larval and juvenile pipefishes were recorded from February to July 2005 (figure 1b) than during the entire period 1958–1972 (during which only 17 individuals were recorded; Coombs 1980, fig. 7a) and sampling intensity has not changed significantly. Figure 1c shows the increase in spatial extent of E. aequoreus larvae and juveniles in CPR samples since 2002. Only one juvenile was sampled in 2002 from the continental shelf southwest of Ireland, whereas in 2005 E. aequoreus appeared in CPR samples as far west as the Mid-Atlantic Ridge. A comparison of average winter, spring and summer SST in the Northeastern Atlantic during the periods 1958–1972 and 2002–2005 (figure 2) shows that the average SST is now up to 0.5°C higher in the region of increased pipefish abundance (figure 1c).
4. Discussion
The snake pipefish, E. aequoreus (figure 1a), is the most oceanic of the Northeast Atlantic species in the pipefish family, whose other members are more usually associated with coastal eelgrass meadows (Vincent et al. 1995) and, in common with other pipefishes, E. aequoreus is a batch spawner. The Northeastern Atlantic distribution of E. aequoreus extends from the Azores to Iceland (Dawson 1986) and, within this range, CPR samples indicate that the planktonic larvae and juveniles of E. aequoreus have increased in abundance over a large spatial area since 2002.
In S. typhle, male egg incubation time is reduced by 23 days on average by a rise in temperature from 10 to 15°C (Ahnesjö 1995) so that at warmer temperatures, males can breed more frequently. This decrease in incubation time makes the OSR less biased towards females, increases the PRR of males (Clutton-Brock & Vincent 1991), and thereby decreases the sexual difference in the PRR, and reduces female–female competition for mates (Ahnesjö et al. 2001). This not only suggests a mechanism through which warmer SST in the Northeastern Atlantic might contribute to an increased abundance of juvenile E. aequoreus in CPR samples, but also suggests how climate change may putatively have a direct impact on mating competition and the dynamics of the sex roles.
Warmer sea temperatures, through their effect upon growth rates, also shorten the duration of the vulnerable larval stage of fishes (Hakala et al. 2003; Martell et al. 2005). If temperature affects larval pipefishes similarly, warmer conditions will mean they are exposed to piscivorous fishes and planktivorous organisms for shorter time. Since mortality through predation in the plankton is size selective (Anderson 1988), small changes in larval growth rates can have a large impact upon survivorship and recruitment (Cushing & Horwood 1994).
In seahorses, warmer conditions also affect the breeding season by advancing its onset and extending its length (Foster & Vincent 2004). This could also contribute to the increased abundance of E. aequoreus in CPR samples (figure 1b). Conceivably, more offspring as a result of changes in the PRR of males, together with an extended breeding season and improved juvenile survival, will improve recruitment and give rise to an increase in the number of reproducing adults. Therefore, changes in SST in the Northeastern Atlantic may influence several life-history stages in E. aequoreus to synergistically increase the abundance of larval pipefishes in the plankton year after year.
In our paper, we have only considered the putative effect of temperature on reproduction and larval survival in E. aequoreus based upon known physiological effects of temperature in closely related taxa. We cannot rule out other environmental changes that might also influence the abundance of E. aequoreus. For example, long-term changes in trophic levels (Pinnegar et al. 2002; Steele 2004) might have reduced predation on juveniles. The importance of temperature on the abundance of the related Nilsson's pipefish, Syngnathus rostellatus, has nevertheless already been noted in the Thames (Power & Attrill 2003). In that estuary, the relationship between S. rostellatus abundance and environmental temperature is nonlinear, decreasing towards the temperature extremes of 11 and 17°C (Power & Attrill 2003), which presumably reflects the thermal niche of this species. Consequently, if warmer SST is responsible for the increased abundance of larval E. aequoreus that we have observed, their increased abundance may eventually prove to be a transitory phenomenon within its present spatial range if North Atlantic SST continues to rise.
Acknowledgments
R.R.K. is a Royal Society University Research Fellow. We thank the owners, masters and crews of the ships for agreeing to tow CPRs and J. Kennedy for providing the SST data. We also thank I. Ahnesjö and another referee for comments on the manuscript.
References
- Ahnesjö I. Temperature affects male and female potential reproductive rates differently in the sex-role reversed pipefish, Sygnathus typhle. Behav. Ecol. 1995;6:229–233. [Google Scholar]
- Ahnesjö I, Kvarnemo C, Merilaita S. Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behav. Ecol. 2001;12:397–401. doi:10.1093/beheco/12.4.397 [Google Scholar]
- Anderson J.T. A review of size-dependent survival during pre-recruit stages of fishes in relation to recruitment. J. North-west Atl. Fish. Sci. 1988;8:55–66. [Google Scholar]
- Batten S.D, et al. CPR sampling: the technical background, materials and methods, consistency and comparability. Prog. Oceanogr. 2003;58:193–215. doi:10.1016/j.pocean.2003.08.004 [Google Scholar]
- Beaugrand G, Reid P.C, Ibañez F, Lindley J.A, Edwards M. Reorganization of Atlantic marine copepod biodiversity and climate. Science. 2002;296:1692–1694. doi: 10.1126/science.1071329. doi:10.1126/science.1071329 [DOI] [PubMed] [Google Scholar]
- Brander K, et al. Changes in fish distribution in the eastern north Atlantic: are we seeing a coherent response to changing temperature? ICES Mar. Sci. Symp. 2003;219:261–270. [Google Scholar]
- Clutton-Brock T.H, Vincent A.C.J. Sexual selection and the potential reproductive rates of males and females. Nature. 1991;351:58–60. doi: 10.1038/351058a0. doi:10.1038/351058a0 [DOI] [PubMed] [Google Scholar]
- Coombs S.H. Continuous plankton records: a plankton atlas of the North Atlantic and North Sea. Supplement 5—young fish, 1948–1972. Bull. Mar. Ecol. 1980;8:229–281. [Google Scholar]
- Cushing D.H, Horwood J.W. The growth and death of fish larvae. J. Plankton Res. 1994;16:291–300. [Google Scholar]
- Dawson C.E. Syngnathidae. In: Whitehead P.J.P, Bauchot M.-L, Hureau J.-C, Nielsen J, Tortonese E, editors. Fishes of the north-eastern Atlantic and the Mediterranean. vol. II. UNESCO; Paris, France: 1986. pp. 628–629. [Google Scholar]
- Foster S.J, Vincent A.C.J. Life history and ecology of seahorses: implications for conservation and management. J. Fish Biol. 2004;65:1–61. doi:10.1111/j.0022-1112.2004.00429.x [Google Scholar]
- Hakala T, Viitasalo M, Rita H, Aro E, Flinkman J, Vuorinen I. Temporal and spatial variation in the growth rates of Baltic herring (Clupea harengus membras L.) larvae during summer. Mar. Biol. 2003;142:25–33. [Google Scholar]
- Hurrell J.W, van Loon H. Decadal variations in climate associated with the North Atlantic Oscillation. Climate Change. 1997;36:301–326. doi:10.1023/A:1005314315270 [Google Scholar]
- Kvarnemo C, Ahnesjö I. Operational sex ratios and mating competition. In: Hardy I, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 366–382. [Google Scholar]
- Leggett W.C, Deblois E. Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth. J. Sea. Res. 1994;32:119–134. doi:10.1016/0077-7579(94)90036-1 [Google Scholar]
- Martell D.J, Keiffer J.D, Trippel E.A. Effects of temperature during early life history on embryonic and larval development and growth in haddock. J. Fish Biol. 2005;66:1558–1575. doi:10.1111/j.0022-1112.2005.00699.x [Google Scholar]
- Palumbi S.R, Martin A, Romano S, McMillan W.O, Stice L, Grabowski G. University of Hawaii Zoology Department; Honolulu, HI: 1991. The simple fool's guide to PCR, Version 2. [Google Scholar]
- Pinnegar J.K, Jennings S, O'Brien C.M, Polunin N.V.C. Long-term changes in the trophic level of the Celtic Sea fish community and fish market price distribution. J. Appl. Ecol. 2002;39:377–390. doi:10.1046/j.1365-2664.2002.00723.x [Google Scholar]
- Power M, Attrill M.J. Long-term trends in the estuarine abundance of Nilsson's pipefish (Sygnathus rostellatus Nilsson) Estuar. Coast. Shelf Sci. 2003;57:325–333. doi:10.1016/S0272-7714(02)00358-X [Google Scholar]
- Quero J.C, Du Buit M.H, Vayne J.J. Les observations de poissons tropicaux et le réchauffement des eaux dans l'Atlantique européen. Oceanol. Acta. 1998;21:345–351. doi:10.1016/S0399-1784(98)80021-2 [Google Scholar]
- Steele J.H. Regime shifts in the ocean: reconciling observations and theory. Prog. Oceanogr. 2004;60:135–141. doi:10.1016/j.pocean.2004.02.004 [Google Scholar]
- Vincent A.C.J, Berglund A, Ahnesjö I. Reproductive ecology of five pipefish species in one eelgrass meadow. Env. Biol. Fishes. 1995;44:347–361. doi:10.1007/BF00008250 [Google Scholar]
- Wilson A.B, Ahnesjö I, Vincent A.J.C, Meyer A. The dynamics of male brooding, mating patterns, and sex roles in pipefishes and seahorses (family Sygnathidae) Evolution. 2003;57:1374–1386. doi: 10.1111/j.0014-3820.2003.tb00345.x. doi:10.1554/02-090 [DOI] [PubMed] [Google Scholar]
- Winters G.H, Wheeler J.P. Environmental and phenotypic factors affecting the reproductive cycle of Atlantic herring. ICES J. Mar. Sci. 1996;53:73–88. doi:10.1006/jmsc.1996.0007 [Google Scholar]