Abstract
Maternal effects are an important source of adaptive variation, but little is known about how they vary throughout ontogeny. We estimate the contribution of maternal effects, sire genetic and environmental variation to offspring body size from birth until 1 year of age in the live-bearing fish Poecilia parae. In both the sexes, maternal effects on body size were initially high in juveniles, and then declined to zero at sexual maturity. In sons, this was accompanied by a sharp rise in sire genetic variance, consistent with the expression of Y-linked loci affecting male size. In daughters, all variance components decreased with time, consistent with compensatory growth. There were significant negative among-dam correlations between early body size and the timing of sexual maturity in both sons and daughters. However, there was no relationship between early life maternal effects and adult longevity, suggesting that maternal effects, although important early in life, may not always influence late life-history traits.
Keywords: maternal effects, offspring size, ontogeny, Y-chromosome, Poecilia parae
1. Introduction
An offspring's phenotype is determined not only by its own genotype and the random environmental conditions it experiences during development, but also by the environment provided by its parents (Mousseau & Fox 1998a). The environment provided by the mother usually contributes considerably more to offspring phenotype than that by the father, and this asymmetry is generally referred to as a maternal effect (Mousseau & Fox 1998a). Recent theoretical (Kirkpatrick & Lande 1989; Cheverud & Moore 1994; Wade 1998) and empirical studies (reviewed in Mousseau & Fox 1998b) have demonstrated that maternal effects can have profound evolutionary implications.
Despite their potential importance, there is surprisingly little known about the biology of maternal effects (Mousseau & Fox 1998a), particularly their persistence across consecutive developmental stages (Wilson & Réale 2006). A common pattern that emerges from empirical studies is that maternal effects are typically large early in development and far less so by the time offspring mature (Bernardo 1996; Heath & Blouw 1998). This finding strongly suggests that the importance of maternal effects declines throughout offspring development. However, because most empirical studies only quantify maternal effects at birth and adulthood, little is known about the importance of maternal effects relative to other causal components of variance (e.g. additive genetic and environmental effects) throughout development. For example, reductions in maternal effects through ontogeny could arise because the relative importance of either random environmental variation and/or additive genetic variance increases across consecutive developmental stages.
Here, we quantify the relative importance of maternal effects (VM), sire genetic variation (VS) and residual environmental variation (VE) to offspring body size throughout maturation in both the sexes of the live-bearing poeciliid fish Poecilia parae. We examine the consequences of these maternal effects on two important life-history parameters: the timing of sexual development and longevity.
2. Material and methods
(a) Fish biology and collection
Poecilia parae is a live-bearing poeciliid fish with internal fertilization. In this genus, offspring develop for 20–30 days within ovarian follicles located inside a vascularized ovary and are independent after birth (Constantz 1989). Wild-caught females produce 4.0±0.3 (mean±s.e.) offspring per brood (n=63, A. K. Lindholm 1999, unpublished). Males are characterized by five colour morphs (red, yellow, blue, parae and immaculata) and an associated size dimorphism (Lindholm et al. 2004). Pedigree analysis has demonstrated that the locus or loci controlling this colour polymorphism are linked to the Y-chromosome (Lindholm et al. 2004). This mode of inheritance is common in poeciliid fishes, with 13 species known to have Y-linked variation in male colour and/or body size (Lindholm & Breden 2002).
In February 2002, we collected 125 males and 125 females from two sites along the Demerera River in Guyana, South America: 6°41.77′ N; 58°12.07′ W and 6°48.06′ N; 58°09.14′ W. All the fishes were air-freighted to Sydney, Australia, where they were kept at 26°C on a 12 h day : night cycle and fed to satiation on brine shrimp once per day, 6 days per week. Offspring were collected daily and reared individually in 1 l plastic containers. These offspring served as the parents in our breeding design.
(b) Breeding design
Our breeding design consisted of 31 sires (four red, six blue, seven yellow, seven parae and seven immaculata), each mated to up to four randomly selected virgin dams. Offspring were collected daily and individually housed in 1 l plastic containers containing 750 ml water and 5 mm gravel, on two adjacent rows of shelves. Containers of newborn offspring were haphazardly stacked, with siblings placed at non-adjacent positions. The positions of containers were then shuffled twice per week to minimize any location effects.
Body size was measured from the anterior tip of the jaw to the posterior end of the caudal peduncle to the nearest 0.5 mm flat against a ruler by the same observer at 2, 4, 6, 8, 12, 16, 20, 28, 36 and 52 weeks of age. Each offspring was visually examined free-swimming in its tank twice per week to assess sexual development. Following Farr & Travis (1986), our index of the onset of sexual maturity was the appearance of pigmented markings on either side of the gonopore in females (see Lindholm et al. 2004) and by the differentiation of the anal fin into the gonopodium in males.
Many matings did not produce viable offspring. Moreover, six offspring from five families were born with skeletal curvature, and thus removed from our dataset. In total, 20 sires, 32 dams and 157 offspring (78 daughters and 79 sons) were available for quantitative genetic analysis.
(c) Statistical analysis
To estimate VS, VM and VE, we modelled offspring body size as 10 age-specific traits (see Wilson et al. 2005) in a nested model (sire+dam within sire) using REML in SPSS (v. 12.0, varcomp routine). Among-dam correlations were estimated using ASREML (http://www.vsn-intl.com/ASReml/). In both the cases, significance was assessed using log-likelihood ratio tests. Asymmetries between sire and dam variance components in this kind of design commonly arise when dam estimates are inflated by maternal effects (Lynch & Walsh 1998). Consequently, we use VDAM−VS as our estimate of maternal effects (VM; Lynch & Walsh 1998). While this calculation assumes that epistasis and dominance are negligible, studies quantifying maternal effects have shown that they are generally much larger than dominance or epistatic effects, making this assumption reasonable (Cheverud & Moore 1994). Since variance commonly increases with the square of the mean, scale effects may limit the utility of directly comparing the magnitudes of (co)variance components across different ages. We therefore scale variance components by the relevant trait phenotypic mean using the coefficient of variation (CV; Houle 1992).
3. Results
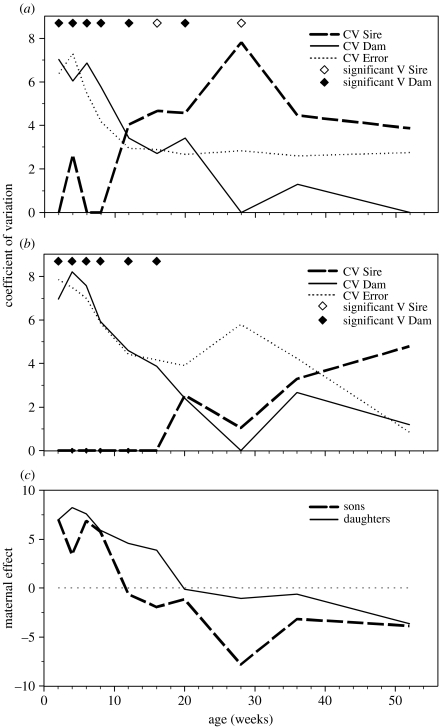
In early development, body size in male (figure 1a) and female (figure 1b) P. parae is strongly influenced by dam variance, which is reflected in the maternal effect (figure 1c). In sons, but not daughters, sire variance increased rapidly after eight weeks (figure 1a,b). In both the sexes, the residual environmental variance decreased during ontogeny. These trends are supported by ANOVA of variance components estimated for each age and sex (table 1). Age of the offspring had a significant effect on all variance components with CVS showing a significant increase with age, whereas all other variance components (CVM, CVE, CVP) significantly decreased with age (table 1). Moreover, sex of the offspring only influenced levels of CVS for body size, being significantly greater in sons than in daughters (table 1).
Figure 1.
The causal components of variance for offspring body size at each age for (a) male and (b) female Poecilia parae. Variances that are significantly greater than zero at each age are represented by closed diamonds (VDAM) and open diamonds (VS). (c) Maternal effects (CVM) for offspring body size at each age for sons and daughters.
Table 1.
The effect of offspring sex and age on the causal components of variation for offspring body size in Poecilia parae.
| source | factor | F(1,1,1,16) | p |
|---|---|---|---|
| CVS | sex | 8.43 | 0.010 |
| age | 16.95 | 0.001 | |
| sex×age | 0.06 | 0.813 | |
| CVM | sex | 1.57 | 0.228 |
| age | 27.43 | 0.001 | |
| sex×age | 0.24 | 0.631 | |
| CVE | sex | 1.91 | 0.186 |
| age | 30.58 | 0.001 | |
| sex×age | 2.92 | 0.107 | |
| CVP | sex | 0.04 | 0.842 |
| age | 24.72 | 0.001 | |
| sex×age | 0.29 | 0.596 |
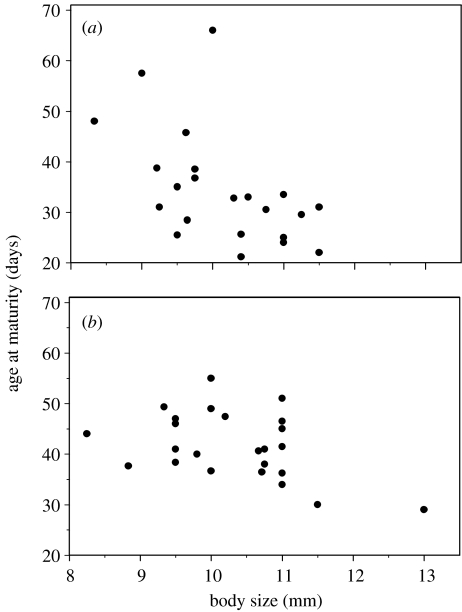
Body size at week 2 was negatively correlated with the index of time to sexual maturity in both sons (mean time to sexual maturity (±s.e.)=32.95±1.15 days; rM=−0.43±0.06, p<0.0001) and daughters (mean time to sexual maturity=40.25±0.86 days; rM=−0.34±0.03, p<0.0001; figure 2). There was, however, no effect of week 2 body size on the longevity of either sons (mean longevity=332.92±18.25 days; rM=0.03±0.02, p=0.13) or daughters (mean longevity=290.19±12.36 days; rM=0.01±0.02, p=0.62).
Figure 2.
Among-dam correlation between offspring body size at two weeks of age and sexual development in (a) male and (b) female Poecilia parae.
4. Discussion
Here, we show that maternal effects are a major determinant of early offspring body size in P. parae and that they decrease from birth onwards. More importantly, we demonstrate that this decline occurs through different pathways in sons and daughters. Furthermore, while we show that maternal effects may have important consequences for life-history traits expressed early in life (e.g. timing of sexual maturity), they do not influence late life-history traits (i.e. adult longevity). This is consistent with previously found associations between large juvenile body size and early maturation (reviewed in Heath & Blouw 1998). Our results, therefore, demonstrate the complex nature of maternal effects and highlight the relevance of ontogenetic changes when examining the evolutionary consequences of maternal effects (Atchley & Zhu 1997; Heath & Blouw 1998; Wilson & Réale 2006).
Several processes may influence changes in causal variance components during ontogeny (reviewed by Wilson & Réale 2006). In daughters, the declining CVM, CVE and CVP through ontogeny, coupled with negligible levels of CVS for body size, is largely consistent with compensatory growth (Wilson & Réale 2006)—the tendency of growth trajectories to converge on a reduced range of phenotypes (Monteiro & Falconer 1966). Compensatory growth is an important mechanism of canalization that buffers the adult phenotype against stressors encountered during early life (Monteiro & Falconer 1966) and appears widespread among vertebrates (Wilson & Réale 2006). As adults, female poeciliids grow much faster than males (Snelson 1989), which would provide greater scope for growth compensation to occur. The dramatic increase of CVS observed in sons, by contrast, probably results from the activation of the Y-chromosome (or associated loci) at about sexual maturity. Similar sex-specific differences in inheritance of body size have been found in other poeciliid species (Kallman 1989).
We quantify maternal effects as the difference between sire and dam variances. Although this is a commonly used measure (see Lynch & Walsh 1998), it assumes that dominance and epistasis variances are negligible. While the allocation of maternal resources to offspring during the formation of yolked eggs and gestation are well known in poeciliids (Constantz 1989), it is possible that our estimates of CVM are inflated by dominance and/or epistasis and affected by the relatively small size of the dataset.
Recently, there has been an increase in the number of studies examining the evolutionary consequences of maternal effects (Mousseau & Fox 1998a). Most studies are, however, phenotypic and only characterize maternal effects at a single point in time, often independent of other variance components. This is surprising, given that our ability to make quantitative genetic predictions about phenotypic evolution is based on such variance components remaining constant across consecutive developmental stages (Lynch & Walsh 1998). Moreover, the evolutionary consequences of maternal effects are seldom examined across different life-history stages. Our finding that maternal effects may have important consequences for life-history traits expressed early in life (i.e. the timing of sexual maturity), but these effects may not persist to later life-history stages (i.e. adult longevity), demonstrates the potential limitations of such an approach and highlights the need for longitudinal studies when examining the adaptive significance of maternal effects.
Acknowledgments
We are grateful for the support of Felix Breden, Peter Kahtoo, Godfrey Bourne, Orrin Clarke and Cecil Persaud. We thank Michael Krützen for his assistance in the field and Charles Fox for his valuable discussions on the ontogeny of maternal effects. This work was funded by the grants from the ARC and UNSW to A.K.L. and R.B. and a NERC Fellowship to J.H.
Footnotes
This research was approved by the Animal Care and Ethics Committee of the University of New South Wales.
References
- Atchley W.R, Zhu J. Developmental quantitative genetics, conditional epigenetic variability and growth in mice. Genetics. 1997;147:765–776. doi: 10.1093/genetics/147.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. Am. Zool. 1996;36:83–105. [Google Scholar]
- Cheverud J.M, Moore A.J. Quantitative genetics and the role of the environment provided by relatives in behavioral evolution. In: Boake C.R.B, editor. Quantitative genetic studies of behavioral evolution. University of Chicago Press; Chicago, IL: 1994. pp. 67–100. [Google Scholar]
- Constantz G.D. Reproductive biology of poeciliid fishes. In: Meffe G.K, Snelson F.F.J, editors. Ecology and evolution of livebearing fishes (Poeciliidae) Prentice Hall; Englewood Cliffs, NJ: 1989. pp. 33–50. [Google Scholar]
- Farr J.A, Travis J. Fertility advertisement by female sailfin mollies, Poecilia latipinna (Pisces: Poeciliidae) Copeia. 1986;1986:467–472. doi:10.2307/1445004 [Google Scholar]
- Heath D.D, Blouw D.M. Are maternal effects in fish adaptive or merely physiological side effects? In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; New York, NY: 1998. pp. 178–201. [Google Scholar]
- Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallman K.D. Genetic control of size at maturity in Xiphophorus. In: Meffe G.K, Snelson F.F Jr, editors. Ecology and evolution of livebearing fishes (Poeciliidae) Prentice Hall; New Jersey, NJ: 1989. pp. 163–184. [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;32:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. doi:10.2307/2409054 [DOI] [PubMed] [Google Scholar]
- Lindholm A, Breden F. Sex chromosomes and sexual selection in poeciliid fishes. Am. Nat. 2002;160:S143–S224. doi: 10.1086/342898. doi:10.1086/342898 [DOI] [PubMed] [Google Scholar]
- Lindholm A.K, Brooks R, Breden F. Extreme polymorphism in a Y-linked sexually selected trait. Heredity. 2004;92:156–162. doi: 10.1038/sj.hdy.6800386. doi:10.1038/sj.hdy.6800386 [DOI] [PubMed] [Google Scholar]
- Lynch W, Walsh B. Sinauer Associates; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Monteiro L.S, Falconer D.S. Compensatory growth and sexual maturity in mice. Anim. Prod. 1966;8:179–192. [Google Scholar]
- Mousseau T.A, Fox C.W. The adaptive significance of maternal effects. Trends Ecol. Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. doi:10.1016/S0169-5347(98)01472-4 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998b. [Google Scholar]
- Snelson F.F.J. Social and environmental control of life history traits in poeciliid fishes. In: Meffe G.K, Snelson F.F.J, editors. Ecology and evolution of live-bearing fishes (Poeciliidae) Prentice Hall; Englewood Cliffs, NJ: 1989. pp. 149–161. [Google Scholar]
- Wade A.J. The evolutionary genetics of maternal effects. In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998. pp. 5–21. [Google Scholar]
- Wilson A.J, Réale D. Ontogeny of additive and maternal genetic effects: lessons from domestic mammals. Am. Nat. 2006;167:E23–E38. doi: 10.1086/498138. doi:10.1086/498138 [DOI] [PubMed] [Google Scholar]
- Wilson A.J, Kruuk L.E.B, Coltman D.W. Ontogenetic patterns in heritable variation for body size: using random regression models in a wild ungulate population. Am. Nat. 2005;166:E177–E192. doi: 10.1086/497441. doi:10.1086/497441 [DOI] [PubMed] [Google Scholar]