Abstract
Recent genetic analyses show that, in social mammals, loss of paternity by breeding males varies with strategies of mate guarding rather than with the degree of polygyny.
Keywords: paternity, breeding systems, mammals
1. Introduction
In a large number of birds and a few mammals, breeding adults form monogamous pairs though either one or both partners sometimes mate outside the pair bond (Kleiman 1977; Birkhead & Moller 1996; Brotherton & Komers 2003). In some socially monogamous (SM) species, monogamy may evolve because females require male assistance in rearing young (Komers & Brotherton 1997; Brotherton & Komers 2003). For example, in a number of birds and some mammals, females are either unable to rear young without male assistance or less successful when they have no partner to assist them (Lack 1968; Ligon 1999; Gubernick & Teferi 2000; Burley & Johnson 2002). However, in other species, males make little or no contribution to provisioning or protecting their offspring and it is unlikely that social monogamy is maintained by any benefits associated with the presence of males (Dunbar 1988; Clutton-Brock 1991; Brotherton & Manser 1997). For example, in dik-dik Madoqua spp., males spend less time with their mates when females have dependent young than at other times of the year and their presence has no obvious benefits to females (Brotherton & Manser 1997; Brotherton & Komers 2003).
Two hypotheses have been made as to why males should defend single females in monogamous species where they do not contribute to parental care (Brotherton & Komers 2003). First, it may not be feasible for a male to defend the range of more than one female because ‘floater’ males swiftly occupy temporarily vacant ranges (Brotherton & Manser 1997). Second, the benefits of polygyny to males may not be as large as they appear if paternity certainty declines as the number of females a male defends increases (Dunbar 1988; Brotherton & Komers 2003). While the first argument applies only to species where females hold separate ranges, the second also applies to species where females form groups and suggests that the benefits of large harem size to males may not increase in proportion to the number of females defended.
Until recently, genetic assessments of paternity in mammals have been too scarce for it to be possible to test the hypothesis that paternity certainty is higher in SM systems than in polygynous or polygynandrous ones. However, the extension of genetic studies to a wider range of species now makes preliminary comparisons feasible. Using genetic estimates of paternity in 24 species, this paper investigates the extent and causes of variation in paternity certainty in mammals and tests the suggestion that paternity certainty is lower in polygynous systems than in SM ones.
2. Material and methods
We extracted estimates of paternity certainty for 24 species (nine primates, four rodents, six carnivores, two bats, one insectivore and two ungulates) from published sources. For each study, we calculated the proportion of young born to resident females that were not fathered by the resident male or (where more than one breeding male was present) by the most dominant resident male as reported by studies (% extra-dominant paternity, EDP; table 1). We classified breeding systems where a single breeding male is present in each group as SM or socially polygynous (SP), allocating systems where breeding groups included several breeding males to a separate category (multi-male, MM). For monogamous systems where occasionally more than one female breeds (as in Lycaon pictus and Suricata suricatta), we used values of EDP for the dominant female alone since subordinates are commonly related to the resident male and breed with members of other groups (Griffin et al. 2003). Available estimates for Helogale parvula (table 1) do not separate data for dominant and subordinate females and are consequently likely to exaggerate EDP. In addition, for SM and SP systems, we distinguished between systems where males were closely associated with breeding females (continuously associated, CA) and those where males were only intermittently associated with the females they defended (intermittently associated, IA). We classified species as CA if females were continuously guarded by males during the period of oestrous (e.g. Madoqua kirkii), or if males were spatially closely associated with females during active periods (e.g. Hapalemur griseus). Species were classified as IA if males did not closely guard females (e.g. Hypogeomys antimena), or if males defended territories overlapping female ranges but foraged separately, so that breeding females were frequently out of sight of the dominant male (e.g. Urocyon littoralis), or if females commonly moved between groups defended by individual males (e.g. Cervus elaphus). We modelled EDP as a function of breeding system and association pattern using linear models with normally distributed errors. EDP was arcsine square-root transformed in all analyses and all estimates shown are back-transformed; standard errors reported are therefore asymmetrical and shown as (estimate−1 s.e., estimate+1 s.e.). The taxa in our sample are widely distributed across multiple clades suggesting that phylogenetic correction is not necessary. However, we also ran analyses incorporating phylogenetic information, using phylogenetic generalized least-squares methods (Martins & Hansen 1997), which gave similar results.
Table 1.
Proportion of offspring not fathered by the dominant or sole male in each group (% EDP) in 24 mammals when paternity has been assessed using genetic techniques. (The sample includes nine primates, four rodents, six carnivores, one insectivore, two bats and two ungulates. Social breeding system: SM, social monogamy; SP, social polygyny; MM, multi-male. Association patterns: CA, continuously associated; IA, intermittently associated. In four species (Alouatta seniculus, Crocidura russula, Hapalemur griseus and Semnopithecus entellus), separate estimates were available for the different breeding systems. We have included these as separate points in our analysis (figure 1), but including only one estimate of %EDP for the predominant breeding system does not affect the outcome of the analysis.)
| species | social breeding system | association pattern between males and females | %EDP | sources |
|---|---|---|---|---|
| Peromyscus californicus | SM | CA | 0 | Ribble (1991) |
| Hypogeomys antimena | SM | IA | 4.2 | Sommer (2003) |
| Marmota marmota | SM | IA | 15.9 | Arnold (1990) and Cohas et al. (2006) |
| Hapalemur griseus | SM | CA | 8.7 | Nievergelt et al. (2002) |
| Cheirogaleus medius | SM | IA | 43.8 | Fietz et al. (2000) |
| Crocidura russula | SM | IA | 0 | Bouteiller & Perrin (2000) |
| Madoqua kirkii | SM | CA | 0 | Brotherton et al. (1997) |
| Urocyon littoralis | SM | IA | 25.0 | Roemer et al. (2001) |
| Lycaon pictus | SM | CA | 10.3 | Girman et al. (1997) |
| Suricata suricatta | SM | CA | 17.0 | Griffin et al. (2003) |
| Helogale parvula | SM | CA | 24.0 | Keane et al. (1994) |
| Hapalemur griseus | SP | CA | 7.1 | Nievergelt et al. (2002) |
| Alouatta seniculus | SP | CA | 0 | Pope (1990) |
| Semnopithecus entellus | SP | CA | 0 | Launhardt et al. (2001) |
| Crocidura russula | SP | IA | 0 | Bouteiller & Perrin (2000) |
| Mirounga angustirostris | SP | IA | 60.0 | Hoelzel et al. (1999) |
| Mirounga leonina | SP | IA | 42.0 | Hoelzel et al. (1999) |
| Artibeus jamaicensis | SP | IA | 45.0 | Ortega et al. (2003) |
| Saccopteryx bilineata | SP | IA | 69.9 | Heckel & von Helversen (2003) |
| Cervus elaphus | SP | IA | 37.8 | Pemberton et al. (unpublished data) |
| Cryptomys hottentotus | MM | — | 44.9 | Bishop et al. (2004) |
| Semnopithecus entellus | MM | — | 43.0 | Launhardt et al. (2001) |
| Alouatta seniculus | MM | — | 7.1 | Pope (1990) |
| Macaca fascicularis | MM | — | 28.9 | de Ruiter et al. (1994) |
| Macaca mulatta | MM | — | 86.7 | Berard et al. (1994) |
| Mandrillus sphinx | MM | — | 26.7 | Wickings et al. (1993) |
| Gorilla beringei beringei | MM | — | 15.0 | Bradley et al. (2005) |
| Eulemur fulvus rufus | MM | — | 33.3 | Wimmer & Kappeler (2002) |
3. Results
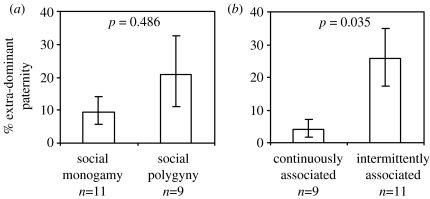
The proportion of offspring fathered by males other than the resident dominant (%EDP) varies widely in both monogamous and polygynous species, ranging from 0 to over 40% in monogamous species and from 0 to over 80% in polygynous and MM societies (see table 1). Contrary to the hypothesis that polygyny is associated with inevitable reductions in paternity certainty, there is no significant difference in %EDP between SM and SP species, though there is a tendency in this direction (figure 1a). In contrast, where one or more females are defended by a single breeding male, %EDP is significantly higher in systems where males are continuously associated with the females they are defending (CA) than in systems where males forage independently or female groups are unstable (IA; figure 1b). In a linear model with %EDP as the response variable and with both breeding system and association pattern as explanatory variables, the estimated difference between categories of breeding system controlling for association pattern was 4.0% (estimate−1 s.e., estimate+1 s.e.=−1.2, 12.3; t17=0.712, p=0.486), while the estimated difference between categories of association pattern controlling for breeding system was 18.3% (8.5, 30.2; t17=2.293, p=0.035). MM systems show levels of %EDP similar to those in uni-male systems where males are not closely associated with females and significantly higher than those found where a single male is continuously associated with one or more females (%EDP in MM species: mean=34.7 (25.8, 44.3), N=8; linear model comparing %EDP of MM, CA and IA: F2,25=5.43, p=0.011; difference in EDP between MM and CA=30.6% (23.5, 34.3), t=−3.09, padjusted=0.005; difference between MM and IA=9.1% (−3.2, 19.6), t=−0.75, padjusted=0.461; p-values are adjusted for multiple comparisons using sequential Bonferroni correction on three comparisons).
Figure 1.
Mean (±1 s.e.) %EDP in species with (a) contrasting breeding systems and (b) contrasting patterns of association between males and females. The same 20 EDP estimates (see table 1) were used in both panels. p-values shown are from a linear model with %EDP as the response variable and with both breeding system and association pattern as explanatory variables (see §3).
4. Discussion
Our analyses provide no evidence of any strong association between %EDP and the nature of breeding systems; the average difference in %EDP between SM and SP systems while controlling for association pattern was small (4%) and not statistically significant. The absence of a consistent difference in %EDP between species where a male defends a single female and species where males defend several females (figure 1a) indicates that polygyny need not reduce paternity certainty compared to that found in SM mammals. This suggests that the principal factor constraining the development of polygyny in mammals where males invest little in their offspring is the dispersion of females. Second, the relatively high levels of %EDP in species where multiple males associate with one or more females (MM) indicate that the presence of additional breeding males usually reduces the mating success of dominant males. Whether dominant males cannot exclude additional males in these species or benefit from their presence and are consequently prepared to share breeding access with them is not yet clear (Clutton-Brock 1998).
The estimates shown in table 1 make it possible for the first time to compare levels of paternity certainty in birds and mammals. Several of the first analyses of paternity certainty in monogamous mammals showed low levels of extra-pair paternity (Ribble 1991; Brotherton et al. 1997), which appeared to contrast with the relatively high levels found in some monogamous birds (Birkhead & Moller 1996). The larger number of genetic studies of mammals now available shows that extra-pair paternity varies widely among monogamous mammals, as it does among monogamous birds (Birkhead & Moller 1992), and suggests that, in both groups, this variation may be related to patterns of association between mating partners.
Acknowledgments
K. Isvaran was supported by a John Stanley Gardiner Fellowship at the University of Cambridge, UK. We thank three anonymous reviewers for their constructive comments on the manuscript.
References
- Arnold W. The evolution of marmot sociality: I. Why disperse late? Behav. Ecol. Sociobiol. 1990;27:229–237. [Google Scholar]
- Berard J.D, Nurnberg P, Epplen J.T, Schmidtke J. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 1994;129:177–201. [Google Scholar]
- Birkhead T.R, Moller A.P. Academic Press; London, UK: 1992. Sperm competition in birds. [Google Scholar]
- Birkhead T.R, Moller A.P. Monogamy and sperm competition in birds. In: Black J.M, editor. Partnerships in birds. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- Bishop J.M, Jarvis J.U.M, Spinks A.C, Bennett N.C, O'Ryan C. Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Mol. Ecol. 2004;13:1217–1229. doi: 10.1111/j.1365-294X.2004.02131.x. doi:10.1111/j.1365-294X.2004.02131.x [DOI] [PubMed] [Google Scholar]
- Bouteiller C, Perrin N. Individual reproductive success and effective population size in the greater white-toothed shrew Crocidura russula. Proc. R. Soc. B. 2000;267:701–705. doi: 10.1098/rspb.2000.1059. doi:10.1098/rspb.2000.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B.J, Robbins M.M, Williamson E.A, Steklis H.D, Steklis N.G, Eckhardt N, Boesch C, Vigilant L. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc. Natl Acad. Sci. USA. 2005;102:9418–9423. doi: 10.1073/pnas.0502019102. doi:10.1073/pnas.0502019102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton P.N.M, Manser M.B. Female dispersion and the evolution of monogamy in the dik-dik. Anim. Behav. 1997;54:1413–1424. doi: 10.1006/anbe.1997.0551. doi:10.1006/anbe.1997.0551 [DOI] [PubMed] [Google Scholar]
- Brotherton P.N.M, Komers P.E. Mate guarding and the evolution of social monogamy in mammals. In: Reichard U.H, Boesch C, editors. Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Brotherton P.N.M, Pemberton J.M, Komers P.E, Malarky G. Genetic and behavioural evidence of monogamy in a mammal, Kirk's dik-dik (Madoqua kirkii) Proc. R. Soc. B. 1997;264:675–681. doi: 10.1098/rspb.1997.0096. doi:10.1098/rspb.1997.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley N.T, Johnson K. The evolution of avian parental care. Phil. Trans. R. Soc. B. 2002;357:241–250. doi: 10.1098/rstb.2001.0923. doi:10.1098/rstb.2001.0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Clutton-Brock T.H. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 1998;13:288–292. doi: 10.1016/s0169-5347(98)01402-5. doi:10.1016/S0169-5347(98)01402-5 [DOI] [PubMed] [Google Scholar]
- Cohas A, Yoccoz N.G, Da Silva A, Goossens B, Allaine D. Extra-pair paternity in the monogamous alpine marmot (Marmota marmota): the roles of social setting and female mate choice. Behav. Ecol. Sociobiol. 2006;59:597–605. doi:10.1007/s00265-005-0086-8 [Google Scholar]
- de Ruiter J.R, Vanhooff J, Scheffrahn W. Social and genetic aspects of paternity in wild long-tailed macaques (Macaca fascicularis) Behaviour. 1994;129:203–224. [Google Scholar]
- Dunbar R.I.M. Croom Helm; London, UK: 1988. Primate social systems. [Google Scholar]
- Fietz J, Zischler H, Schwiegk C, Tomiuk J, Dausmann K.H, Ganzhorn J.U. High rates of extra-pair young in the pair-living fat-tailed dwarf lemur Cheirogaleus medius. Behav. Ecol. Sociobiol. 2000;49:8–17. doi:10.1007/s002650000269 [Google Scholar]
- Girman D.J, Mills M.G.L, Geffen E, Wayne R.K. A molecular genetic analysis of social structure, dispersal, and interpack relationships of the African wild dog (Lycaon pictus) Behav. Ecol. Sociobiol. 1997;40:187–198. doi:10.1007/s002650050332 [Google Scholar]
- Griffin A.S, Pemberton J.M, Brotherton P.N.M, McIlrath G, Gaynor D, Kansky R, O'Riain J, Clutton-Brock T.H. A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta) Behav. Ecol. 2003;14:472–480. doi:10.1093/beheco/arg040 [Google Scholar]
- Gubernick D.J, Teferi T. Adaptive significance of male parental care in a monogamous mammal. Proc. R. Soc. B. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. doi:10.1098/rspb.2000.0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel G, Von Helversen O. Genetic mating system and the significance of harem associations in the bat Saccopteryx bilineata. Mol. Ecol. 2003;12:219–227. doi: 10.1046/j.1365-294x.2003.01722.x. doi:10.1046/j.1365-294X.2003.01722.x [DOI] [PubMed] [Google Scholar]
- Hoelzel A.R, Le Boeuf B.J, Reiter J, Campagna C. Alpha-male paternity in elephant seals. Behav. Ecol. Sociobiol. 1999;46:298–306. doi:10.1007/s002650050623 [Google Scholar]
- Keane B, Waser P, Creel S, Creel N, Elliot L, Minchilla D. Subordinate reproduction in dwarf mongooses. Anim. Behav. 1994;47:289–294. doi:10.1006/anbe.1994.1008 [Google Scholar]
- Kleiman D.G. Monogamy in mammals. Q. Rev. Biol. 1977;52:39–69. doi: 10.1086/409721. doi:10.1086/409721 [DOI] [PubMed] [Google Scholar]
- Komers P.E, Brotherton P.N.M. Female space use is the best predictor of monogamy in mammals. Proc. R. Soc. B. 1997;264:1261–1270. doi: 10.1098/rspb.1997.0174. doi:10.1098/rspb.1997.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack D. Methuen & Co; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Launhardt K, Borries C, Hardt C, Epplen J.T, Winkler P. Paternity analysis of alternative male reproductive routes among the langurs (Semnopithecus entellus) of Ramnagar. Anim. Behav. 2001;61:53–64. doi: 10.1006/anbe.2000.1590. doi:10.1006/anbe.2000.1590 [DOI] [PubMed] [Google Scholar]
- Ligon J.D. Oxford University Press; Oxford, UK: 1999. The evolution of avian breeding systems. [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. doi:10.1086/286013 [Google Scholar]
- Nievergelt C.M, Mutschler T, Feistner A.T.C, Woodruff D.S. Social system of the alaotran gentle lemur (Hapalemur griseus alaotrensis): genetic characterization of group composition and mating system. Am. J. Primatol. 2002;57:157–176. doi: 10.1002/ajp.10046. doi:10.1002/ajp.10046 [DOI] [PubMed] [Google Scholar]
- Ortega J, Maldonado J.E, Wilkinson G.S, Arita H.T, Fleischer R.C. Male dominance, paternity, and relatedness in the Jamaican fruit-eating bat (Artibeus jamaicensis) Mol. Ecol. 2003;12:2409–2415. doi: 10.1046/j.1365-294x.2003.01924.x. doi:10.1046/j.1365-294X.2003.01924.x [DOI] [PubMed] [Google Scholar]
- Pope T.R. The reproductive consequences of cooperation in the red howler monkey: paternity exclusion in multi-male and single-male troops using genetic markers. Behav. Ecol. Sociobiol. 1990;27:439–446. doi:10.1007/BF00164071 [Google Scholar]
- Ribble D.O. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 1991;29:161–166. doi:10.1007/BF00166397 [Google Scholar]
- Roemer G.W, Smith D.A, Garcelon D.K, Wayne R.K. The behavioural ecology of the island fox (Urocyon littoralis) J. Zool. 2001;255:1–14. doi:10.1017/S0952836901001066 [Google Scholar]
- Sommer S. Social and reproductive monogamy in rodents: the case of the Malagasy giant jumping rat (Hypogeomys antimena) In: Reichard U.H, Boesch C, editors. Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press; Cambridge, UK: 2003. [Google Scholar]
- Wickings E.J, Bossi T, Dixson A.F. Reproductive success in the mandrill, Mandrillus sphinx: correlations of male dominance and mating success with paternity, as determined by DNA fingerprinting. J. Zool. 1993;231:563–574. [Google Scholar]
- Wimmer B, Kappeler P.M. The effects of sexual selection and life history on the genetic structure of redfronted lemur, Eulemur fulvus rufus, groups. Anim. Behav. 2002;64:557–568. doi:10.1006/anbe.2002.4003 [Google Scholar]