Abstract
Atlantic cod (Gadus morhua L.) with the HbI-(2/2) haemoglobin phenotype have a higher blood oxygen affinity at low temperatures and a lower routine metabolic rate than individuals with the HbI-(1/1) phenotype. In the present study, muscle structure was found to be related to haemoglobin phenotype in a coastal population of Atlantic cod from the Saltenfjord region of Northern Norway. The maximum number of fast muscle fibres (FNmax) was reached at approximately 39 cm fork length and was 15% greater in the HbI-(1/1) than in the HbI-(2/2) phenotypes whereas the average fibre diameter for fish of the same fork length was significantly lower. Theoretically, the higher oxygen affinity of the HbI-(2/2) phenotype in the cold water of northern latitudes could have resulted in a relaxation of diffusional constraints at the level of individual muscle fibres, permitting the observed increase in fibre diameter. The results support the optimal fibre number hypothesis which envisages a trade-off between diffusional constraints and the energy cost of maintaining ionic homeostasis with fewer larger diameter muscle fibres in the HbI-(2/2) phenotype contributing to a lower routine metabolic rate.
Keywords: muscle fibre recruitment, biogeographic patterning, biodiversity
1. Introduction
Haemoglobin from Atlantic cod Gadus morhua occurs as two homozygous [HbI-(1/1), HbI-(2/2)] and one heterozygous phenotype [HbI-(1/2)] based on agar gel-electrophoresis (Sick 1961). In the North Atlantic, HbI-(2/2) is present at higher frequencies in the north whereas HbI-(1/1) is dominant in the south (Sick 1965). Haemoglobin phenotypes comprise combinations of four alpha and beta globin genes (Verde & diPrisco 2004). The HbI-(2/2) phenotype has a higher oxygen binding affinity than the HbI-(1/1) phenotype below 10 °C (Brix et al. 1998). At temperatures above 14 °C, and at some blood pHs, the HbI-(1/1) phenotype has a higher oxygen affinity than the HbI-(2/2) phenotype with intermediate values found in the HbI-1/2 phenotype (Karpov & Novikov 1981). Preferred body temperature is significantly lower for HbI-(2/2; 8.2 °C) than HbI-(1/1; 15.4 °C) phenotypes (Petersen & Steffensen 2003). At 6 °C, competitive feeding behaviour was higher for the HbI-(2/2) than HbI-(1/1) phenotypes (Salvanes & Hart 2000) and was correlated with a higher growth rate and age at first spawning (Mork et al. 1984; Jørstad & Nævdal 1994). In contrast, the aerobic scope for activity and maximum swimming speed were significantly higher for the HbI-(1/1) than the HbI-(2/2) phenotypes (Petersen & Stefensen 2003). Thus at northern latitudes Atlantic cod with the HbI-(1/1) phenotype appears to live more active life-styles than cod with the HbI-(2/2) phenotype, but mature later and grow more slowly.
The maximum number (FNmax) of fast fibres is positively correlated with ultimate body size (Johnston et al. 2003a). Dwarfism in landlocked populations of Arctic charr (Salvelinus alpinus; Johnston et al. 2004) and Atlantic salmon (Salmo salar; Johnston et al. 2005) has led to a rapid loss of muscle fibres. The radiation of Antarctic notothenioid fishes resulted in a relaxation of diffusional constraints due to climatic cooling, and phylogenetic analyses demonstrated a dramatic reduction in FNmax relative to the ancestral condition (Johnston et al. 2003a). This led us to advance the optimal fibre number hypothesis, which provides a physiological explanation for changes in FNmax with body size and environment (Johnston et al. 2003a, 2004, 2005). The energy cost of maintaining ionic homeostasis is thought to represent 20–40% of routine metabolic rate (Jobling 1994). Theoretically, as the surface to volume ratio of muscle sarcolemma decreases so does the passive leak of ions across the membrane and hence the cost of maintaining ionic homeostasis involving ATP-dependent pumps. According to our hypothesis there is an optimal fibre number for a given body size and environment which minimizes the energy costs of maintaining ionic homeostasis across the surface area of the muscle sarcolemma. Fibres that exceed the optimum diameter will incur the penalty of diffusional limitation for oxygen and other metabolites while smaller diameter fibres have an unnecessarily high routine metabolic rate, limiting behavioural flexibility. In the case of Atlantic cod living in the same northern environment the optimal fibre number hypothesis predicts that the HbI-(1/1) phenotype with a faster lifestyle would have smaller diameter fibres and a higher FNmax than the HbI-(2/2) phenotype. The aim of the present study was to test this prediction for a cod population from the Saltenfjord region of northern Norway.
2. Material and methods
Atlantic cod (G. morhua L.; 29.0–101.4 cm fork-length (FL, length from the tip of the snout with closed mouth to the centre fork of the tail), 250–10 415 g n=49] were caught between June and August 2005 in the Saltenfjord region of Northern Norway (latitude 67°17′ N) using a beam trawl and line fishing. Fishes were killed by a blow to the head and pithing. Haemoglobin phenotypes were determined by agar gel electrophoresis (Fyhn et al. 1994). The PanI locus was used a genetic marker to distinguish between coastal and Arctic cod because these populations might also differ in muscle fibre number. Genetic divergence at the pantophysin (PanI) locus was determined using a PCR-based assay to score polymorphisms (Fevolden & Pogson 1997).
Frozen sections (7 μm) were prepared from 6 to 12 muscle blocks/fish at 0.7 FL, sampling all the fast muscle on one side of the trunk. Fast and slow fibres were distinguished using the S-58 myosin antibody (Johnston et al. 2004). One hundred randomly selected fast muscle fibres were digitized per block and fibre number and diameter estimated as previously described (Johnston et al. 1999). Non-parametric statistical techniques were used to fit smoothed probability density functions (pdfs) to the measured diameters using a kernel function (Bowman & Azzalini 1997; smoothing coefficient h=0.105). The non-parametric Kolmogorov–Smirnov two-sample test statistic was used to test the null hypothesis that pdfs of fibre diameter for HbI-(1/1) and HbI-(2/2) phenotypes were equal over all diameters. FNmax was analysed in a general linear model ANOVA with haemoglobin phenotype and PanI genotypes as fixed factors. For average fibre diameter, fork length was included as a covariate.
3. Results
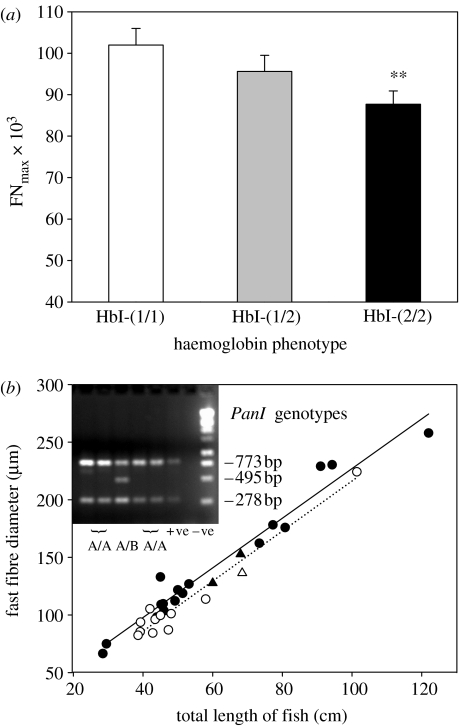
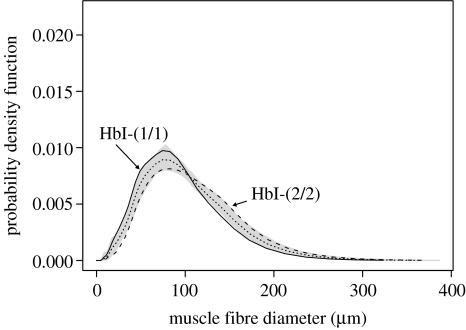
Muscle fibre recruitment ceased at around 39 cm fork length. The two smallest fish sampled had not stopped recruiting fast muscle fibres and were excluded from further analysis. There were 12 HbI-(1/1), 19 HbI-(1/2) and 18 HbI-(2/2) haemoglobin phenotypes. Forty three fish were the PanI-(A/A) genotype and six fish the PanI-(A/B) genotype (figure 1b). FNmax was different between haemoglobin phenotypes (F2,46=3.3, p=0.046), but not PanI genotypes. FNmax was 15% greater for the HbI-(1/1) than the HbI-(2/2) phenotype (figure 1a, p<0.01; Tukey's post hoc test). The maximum fibre diameter reached 500 μm in the largest fish, a HbI-(2/2) phenotype (data not shown). Fish with the HbI-(1/1) phenotype had smaller average diameters than for the HbI-(2/2) phenotype for a given fork length (figure 1b; F2,49=3.41, p=0.042). Fibre diameter was not different between PanI genotypes. Figure 2 illustrates the pdf of fibre diameter for five fish from each of the HbI-(1/1) and HbI-(2/2) phenotypes matched for fork length (see legend). The right-hand side pdf for the HbI-(1/1) phenotype (solid line) was shifted to the left relative to the HbI-(2/2) phenotype (dashed line) whereas the right-hand side of the distribution showed a shift to the right for the HbI-(2/2) relative to the HbI-(1/1) phenotype (p<0.02; Kolmogorov–Smirnoff test).
Figure 1.
(a) Maximum number and (b) average diameter of fast muscle fibres in Atlantic cod (Gadus morhua) with different haemoglobin phenotypes. In (a) mean+s.e., 12 HbI-(1/1), 19 HbI-(1/2) and 18 HbI-(2/2) phenotypes, **indicates significant difference p<0.01. In (b) HbI-(1/1) phenotypes (open symbols), HbI-(2/2) phenotypes (closed symbols), PanI-(A/A) genotypes (circles), PanI-(A/B) genotypes (triangles). First order regression lines were fitted: HbI-2/2 (solid line), HbI-1/1 (dotted line). Insert shows a typical RT-PCR gel for PanI genotypes with positive and negative controls.
Figure 2.
Probability density functions of fibre diameter for five HbI-(1/1) (solid line; 45.3±1.0 cm fork length) and five HbI-(2/2) (dashed line; 45.8±0.9 fork length) haemoglobin phenotypes (mean±s.e.). The dotted line shows the average pdf of fibre diameters across all genotypes and the shaded area 100 bootstrap values of the combined population of diameters.
4. Discussion
Numerous stocks of Atlantic cod (G. morhua L) occur in the Northeast Atlantic and Baltic Sea with different life-history characteristics and migration patterns (Brander 1994). In northern Norway, two main groups can be distinguished, coastal cod and northeast Arctic cod. The latter migrate from feeding areas in the Barents Sea and near Svalbard to spawning areas on the Norwegian coast, most importantly the Lofoten Islands (Brander 1994). The temperature experienced during early life stages can influence the number of fast muscle fibres recruited (Johnston et al. 2003b). To interpret our results it is important to consider whether the fish studied are likely to come from the same spawning stock. Pantophysin is a useful genetic marker for stock discrimination with the PanIA and PanIB alleles occurring at higher frequencies in costal and northeast Arctic cod, respectively (Fevolden & Pogson 1997; Case et al. 2005). Our fish were caught after the northern migration of post-spawning NE Arctic cod and were genotyped PanI-(A/A) or PanI-(A/B) suggesting they come from a coastal spawning population. Although some admixing of populations spawning in different local environments may have occurred, it is highly unlikely that developmental plasticity could explain our results (figure 1a,b).
Mitochondria are present in the central core of fast muscle fibres indicating a requirement for adequate oxygen diffusion during recovery metabolism (Archer & Johnston 1991; Johnston et al. 2004). Factors that affect tissue perfusion such as haemoglobin properties, blood flow and the number and distribution of capillaries will act to determine the limits for diffusion at the level of individual muscle fibres (Egginton et al. 2002). Theoretically, the appearance of a haemoglobin phenotype with a higher oxygen affinity could have allowed a relaxation of diffusional constraints due to a higher partial pressure of oxygen at the fibre surface (Hill 1965). In the present study, there is clear evidence that fibre diameters are larger for the HbI-(2/2) than the HbI-(1/1) phenotype (figures 1b and 2), which would theoretically reduce the energetic costs of ionic homeostasis by decreasing the surface to volume ratio of the sarcolemmal membrane and contribute to the observed reduction in routine metabolic rate. The increase in fibre diameter allows a decrease in FNmax as now fewer muscle fibres are required to reach the maximum body size; representing the trade-off inherent in the optimal fibre number hypothesis. It appears mutations in a few globin-encoding genes have led to changes in tissue structure, physiology, behaviour and life-history strategies with profound consequences for the biogeographic patterning of the species.
Acknowledgments
This work was supported by Norwegian Research Council grant No. 159672 to O.A. The S58 monoclonal antibody used was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. We thank Professor Christel Solberg, Ørjan Hagen and Vera Johnston for their help in obtaining the fish and with sampling.
References
- Archer S.D, Johnston I.A. Density of cristea and distribution of mitochondria in the slow muscle fibres of Antarctic fish. Physiol. Zool. 1991;64:242–258. [Google Scholar]
- Bowman A.W, Azzalini A. Applied smoothing techniques for data analysis. The Kernel approach with S-Plus illustrations. Oxford University Press; Oxford, UK: 1997. [Google Scholar]
- Brander K, editor. Spawning and life history information for North Atlantic cod stocks. ICES Cooperative Research Report 205. ICES; Copenhagen, Denmark: 1994. [Google Scholar]
- Brix O, Forås, Strand I. Genetic variation and functional properties of Atlantic cod haemoglobins: introducing a modified tonometric method for studying fragile haemoglobins. Comp. Biochem. Physiol. 1998;119A:575–583. doi: 10.1016/s1095-6433(97)00469-8. [DOI] [PubMed] [Google Scholar]
- Case R.A, Hutchinson W.F, Hauser L, Van Oosterhout C, Carvalho G.R. Macro- and micro-geographic variation in pantphysin (PanI) allele frequencies in NE Atlantic cod Gadus morhua. Mar. Ecol. Progr. Ser. 2005;301:267–278. [Google Scholar]
- Egginton S, Skilbeck C, Hoofd L, Calvo J, Johnston I.A. Peripheral oxygen transport in the skeletal muscle of Antarctic and sub-Antarctic notothenioid fish. J. Exp. Biol. 2002;205:769–779. doi: 10.1242/jeb.205.6.769. [DOI] [PubMed] [Google Scholar]
- Fevolden S.E, Pogson G.H. Genetic divergence at the synaptophysin (Syp I) locus among Norwergian coastal and north-east Arctic populations of Atlantic cod. J. Fish Biol. 1997;51:895–908. [Google Scholar]
- Fyhn U.E.H, Brix O, Nævdal G, Johansen T. New variants of the haemoglobins of Atlantic cod: a tool for discriminating between coastal and Arctic cod populations. ICES Mar. Sci. Symp. 1994;198:666–670. [Google Scholar]
- Hill A.V. Trials and trails in physiology. ch. 6. Edward Arnold; London, UK: 1965. pp. 208–241. [Google Scholar]
- Jobling M. Chapman and Hall; London, UK: 1994. Fish bioenergetics. [Google Scholar]
- Johnston I.A, Strugnell G, McCracken M.L, Johnstone R.R. Muscle growth and development in normal-sex-ratio and all-female diploid and triploid Atlantic salmon. J. Exp. Biol. 1999;202:1991–2016. doi: 10.1242/jeb.202.15.1991. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Fernandez D, Calvo J, Vieria V.L.A, North T.W, Abercromby M, Garland T., Jr Reduction in muscle fibre number during the adaptive radiation of Notothenioid fishes: a phylogenetic perspective. J. Exp. Biol. 2003a;206:2595–2609. doi: 10.1242/jeb.00474. doi:10.1242/jeb.00474 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Manthri S, Alderson R, Smart A, Campbell P, Nickell D, Robertson B, Paxton C.G.M, Burt M.L. Freshwater environment affects growth rate and muscle fibre recruitment in seawater stages of Atlantic salmon (Salmo salar L.) J. Exp. Biol. 2003b;206:1337–1351. doi: 10.1242/jeb.00262. doi:10.1242/jeb.00262 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Abercromby M, Vieira V.L.A, Sigursteindóttir R.J, Kristjánsson B.K, Sibthorpe D, Skúlason S. Rapid evolution of muscle fibre number in post-glacial populations of Arctic charr Salvelinus alpinus. J. Exp. Biol. 2004;207:4343–4360. doi: 10.1242/jeb.01292. doi:10.1242/jeb.01292 [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Abercromby M, Anderson Ø. Loss of muscle fibres in a landlocked dwarf Atlantic salmon population. Biol. Lett. 2005:1–4. doi: 10.1098/rsbl.2005.0377. doi:10.1098/rsbl.2005.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørstad E.J, Nævdal G. Studies on associations between genotypes and growth rate in juvenile cod. ICES Mar. Sci. Symp. 1994;198:666–670. [Google Scholar]
- Karpov A.K, Novikov G.C. Hemoglobin alloforms in cod Gadus morhua (gadiformes, Gadidae). Their functional characteristics and occurrence in populations. J. Ichthyol. 1981;6:45–49. [Google Scholar]
- Mork J, Giskeødegård R, Sundes G. The haemoglobin polymorphism in Atlantic cod (Gadus morhua L.): genotypic difference in somatic growth and in maturing age in natural populations. Flødevigen rapportser. 1984;1:721–732. [Google Scholar]
- Petersen M.F, Steffensen J.F. Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia. J. Exp. Biol. 2003;206:359–364. doi: 10.1242/jeb.00111. doi:10.1242/jeb.00111 [DOI] [PubMed] [Google Scholar]
- Salvanes A.G.V, Hart P.J.B. Is individual variation in competitive performance of reared juvenile cod influenced by haemoglobin genotype? Sarsia. 2000;85:265–274. [Google Scholar]
- Sick K. Haemoglobin polymorphism in fishes. Nature. 1961;192:894–896. doi: 10.1038/192894a0. [DOI] [PubMed] [Google Scholar]
- Sick K. Haemoglobin polymorphism of cod in the North Sea and the North Atlantic Ocean. Hereditas. 1965;54:49–69. doi: 10.1111/j.1601-5223.1965.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Verde C, diPrisco G. Structure/function and phylogeny of haemoglobins of polar fishes. Micron. 2004;35:77–80. doi: 10.1016/j.micron.2003.10.023. doi:10.1016/j.micron.2003.10.023 [DOI] [PubMed] [Google Scholar]