Abstract
Harvesting represents a major source of mortality in many deer populations. The extent to which harvesting is selective for specific traits is important in order to understand contemporary evolutionary processes. In addition, since such data are frequently used in life-history studies, it is important to know the pattern of selectivity as a source of bias. Recently, it was demonstrated that different hunting methods were selected for different weights in red deer (Cervus elaphus), but little insight was offered into why this occurs. In this study, we show that foreign trophy stalkers select for larger antlers when hunting roe deer (Capreolus capreolus) than local hunters, but that close to half of the difference in selectivity was due to foreigners hunting earlier in the season and in locations with larger males. The relationship between antler size and age was nevertheless fairly similar based on whether deer was shot by foreign or local hunters.
Keywords: antlers, Capreolus capreolus, life history, trophy stalking, management, selection
1. Introduction
Most populations of large herbivores are harvested. A better understanding of the harvesting process is required for two main reasons. Firstly, harvesting represents a major source of mortality in many deer populations (Langvatn & Loison 1999). Even though the dynamic effect of harvesting is well studied, selective harvesting may also favour specific traits such as horn size (Coltman et al. 2003). Secondly, since data derived from harvesting are frequently used in research, it is important to understand the harvesting process as a potential source of bias (Martínez et al. 2005). Clearly, trophy hunting is expected to be selective on specific traits, but cultural traditions in hunting and selectivity vary considerably (Milner et al. 2006).
Recently, Martínez et al. (2005) found that the body weight and age relationship for red deer (Cervus elaphus) in Spain varied depending on whether data are derived from trophy stalking, by catch, management hunt or drive hunt (montería); the main difference being between trophy stalking and other methods. They provided little insight into how differential selectivity arises. Such variation may be directly linked to hunting method, since motivation differs and during drive hunting, there is less time and opportunity to select than during trophy stalking (Martínez et al. 2005). However, such variation might arise also because trophy hunters (often paying high sums) either hunt before other hunters (thus depleting the best trophies) or are allowed to hunt in better areas.
We use antler mass of roe deer (Capreolus capreolus) males, a trait that does not vary over the hunting season (in contrast to body mass), to explore variation in selectivity between foreign trophy stalkers and local hunters (more often using drive hunts and ‘sit-and-wait’ methods) in Poland. The selectivity-hunter method hypothesis (Martínez et al. 2005) suggests that motivation to take a male with large antlers and selectivity at the time of shooting is the most important. We test the alternative hypotheses that selectivity arises due to: (i) depletion (time component; the selectivity–depletion hypothesis) or (ii) spatial variation in where to hunt (the selectivity–honey-pot hypothesis). We demonstrate that the hunter type had a predictable influence on the relationship between age and antler mass, and that close to half of this was due to variation owing to timing of the hunt and where to hunt. All the three processes were thus supported.
2. Material and methods
(a) Study area
The study was carried out in the experimental area (approx. 150 km2) of the Polish Hunting Association Research Station at Czempiń, western Poland (52°08′ N; 16°44′ E), a typical farmland region (70% arable fields) with cereals as the main crop. Roe deer live mainly on arable fields, sporadically using mid-field woodland patches (less than 20 ha) as resting sites. High densities are also registered in a few small (50–300 ha) pine forests. Roe deer from these forests often use neighbouring agricultural lands as an evening–morning feeding area (Bresinski 1982).
(b) Data on harvested roe deer
We obtained data on antler mass from 2172 roe deer males harvested between 1965 and 2005.We only considered ‘typical antlers’ (excluding individuals shot outside the hunting season), thus ending up with a sample size of 1434 shot by foreign hunters and 528 shot by local hunters. Animals were aged by tooth wear. This method is not highly reliable especially for older ages (Mysterud & Østbye 2006), but since the same method was used for all data, it is unlikely to cause bias between local and foreign hunters. For each individual, the date of culling and distance from the nearest forest (i.e. woodland patch>20 ha) was noted. Hunting occurred from the beginning of June to 20 October during the 1960s and 1970s, and recently from 11 May to the end of September.
(c) Statistical analyses
We used a logarithmic transformation of antler mass to get residuals with constant variance. We used linear models after initial use of additive models (AM) with smoothing splines to ensure that predictors were linearly related to the response variables. For age, we tried both polynomial and age as categorical, and modelling yielded similar results irrespective of the way in which age was modelled. Both factors Julian date of harvest and distance to forest were nonlinearly related to (ln) antler mass. Based on the AM plotting, a third-order polynomial seemed appropriate to model the effect of Julian date, while there was a marked threshold at ca 1500 m for the effect of distance from forest. We verified these parametrizations in the model-selection procedure. We entered a ‘year’ term (continuous), as the body mass of roe deer increased over time in this area (Mysterud et al. submitted).
We used the Akaike information criterion (AIC; Crawley 2002) to select the most parsimonious model. We used the small-sample correction (AICc) and retained the model with the lowest AICc value for parameter estimation. We used the manual selection procedures, since a third-order polynomial may give a much better fit than a first-order (i.e. linear) polynomial, but a second-order may not (cf. the effect of Julian date). Analyses were done in S-Plus v. 6.2 (Crawley 2002).
We used a χ2-test to compare whether age of male, place and time of harvest differed between local and foreign hunters.
3. Results
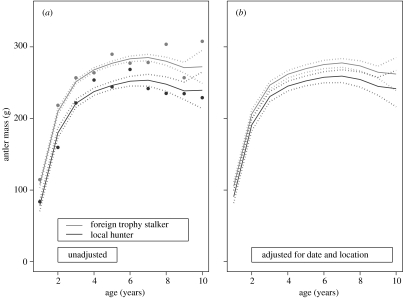
Antler mass was consistently larger for deer shot by foreign trophy stalkers than local hunters (figure 1a). Although the interaction term between age and hunter type entered the final model (tables 1 and 2), the pattern of antler size versus age was very similar irrespective of whether data were derived from local or foreign hunters (figure 1a).
Figure 1.
The relationship between age and antler size of roe deer depending on whether data are derived from animals harvested by foreign or local hunters. (a) Predicted antler size unadjusted for other factors except year (with mean values of raw data for each age class as points). (b) Predicted antler size adjusted for date of shooting and location.
Table 1.
Model selection performed on roe deer (ln) antler size. (x, factor included; AIC, Akaike information criterion; AICc, AIC corrected for sample size; K, number of parameters; ‘hunter type’, foreign trophy stalkers versus local hunters.)
| hunter type | age | (age)2 | (age)3 | (age)4 | (age)5 | hunter: age | year (continous) | Julian date | (Julian date)2 | (Julian date)3 | distance to forest | (distance to forest)2 | distance to forest (0–1500 m) | distance to forest (>1500 m) | hunter×Julian date | hunter× distance to forest | AIC | K | AICc | ΔAICc | AICc weights |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | x | x | x | x | x | x | x | x | x | x | x | x | 1875.624 | 14 | 1875.833 | 0.000 | 0.436 | ||||
| x | x | x | x | x | x | x | x | x | x | x | x | x | 1876.402 | 14 | 1876.611 | 0.778 | 0.296 | ||||
| x | x | x | x | x | x | x | x | x | x | x | x | x | x | 1876.579 | 15 | 1876.818 | 0.985 | 0.266 | |||
| x | x | x | x | x | x | x | x | x | x | x | x | 1887.292 | 13 | 1887.473 | 11.640 | 0.001 | |||||
| x | x | x | x | x | x | x | x | x | x | x | x | x | 1888.996 | 14 | 1889.205 | 13.372 | 0.001 | ||||
| x | x | x | x | x | x | x | x | x | x | x | 1916.216 | 12 | 1916.371 | 40.538 | 0.000 | ||||||
| x | x | x | x | x | x | x | x | 1919.203 | 9 | 1919.292 | 43.459 | 0.000 | |||||||||
| x | x | x | x | x | x | x | x | x | 1921.186 | 10 | 1921.295 | 45.462 | 0.000 | ||||||||
| x | x | x | x | x | x | x | x | x | x | 1922.553 | 11 | 1922.684 | 46.851 | 0.000 | |||||||
| x | x | x | x | x | x | x | 1927.201 | 8 | 1927.273 | 51.440 | 0.000 | ||||||||||
| x | x | x | x | x | x | 1935.011 | 7 | 1935.067 | 59.234 | 0.000 | |||||||||||
| x | x | x | x | x | 1970.910 | 6 | 1970.952 | 95.119 | 0.000 | ||||||||||||
| x | x | x | x | 2092.953 | 5 | 2092.983 | 217.150 | 0.000 | |||||||||||||
| x | x | x | 2477.447 | 4 | 2477.467 | 601.634 | 0.000 |
Table 2.
Analysis of roe deer antler size as a function of age depending on whether data are derived from foreign trophy stalkers and local hunters (‘hunter type’) before and after adjusting for time and location to hunt. For distance to forest, a piecewise linear regression was used.
| parameter | l.s. estimate | s.e. | lower 95% CI | upper 95% CI |
|---|---|---|---|---|
| (a) no adjustment (r2=0.467) | ||||
| intercept | −12.9565 | 2.5968 | −18.1501 | −7.7629 |
| hunter type | −0.2109 | 0.0381 | −0.2871 | −0.1347 |
| age | 5.4920 | 0.8203 | 3.8514 | 7.1326 |
| (age)2 | −1.6670 | 0.3231 | −2.3132 | −1.0208 |
| (age)3 | 0.2490 | 0.0589 | 0.1312 | 0.3668 |
| (age)4 | −0.0181 | 0.0050 | −0.0281 | −0.0081 |
| (age)5 | 0.0005 | 0.0002 | 0.0001 | 0.0009 |
| year | 0.0056 | 0.0013 | 0.0030 | 0.0082 |
| hunter type: age | 0.0218 | 0.0069 | 0.0080 | 0.0356 |
| (b) adjusting for time and space (r2=0.484) | ||||
| intercept | −8.9583 | 3.1167 | −15.1917 | −2.7249 |
| hunter type | −0.1792 | 0.0385 | −0.2562 | −0.1022 |
| age | 5.4353 | 0.8090 | 3.8173 | 7.0533 |
| (age)2 | −1.6429 | 0.3186 | −2.2801 | −1.0057 |
| (age)3 | 0.2436 | 0.0581 | 0.1274 | 0.3598 |
| (age)4 | −0.0176 | 0.0050 | −0.0276 | −0.0076 |
| (age)5 | 0.0005 | 0.0002 | 0.0001 | 0.0009 |
| year | 0.0055 | 0.0013 | 0.0029 | 0.0081 |
| Julian date | −0.0581 | 0.0247 | −0.1075 | −0.0087 |
| (Julian date)2 | 0.0003 | 0.0001 | 0.0001 | 0.0005 |
| (Julian date)3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| distance to forest (0–1500 m) | 0.0001 | 0.0000 | 0.0001 | 0.0001 |
| distance to forest (>1500 m) | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| hunter type: age | 0.0209 | 0.0068 | 0.0073 | 0.0345 |
We then explored how other factors such as date of harvesting and location may affect antler size, and how much hunter type affected the antler size after adjusting for these factors. The most parsimonious model explained 49.2% of the variation in (ln) antler mass and included up to a third-order term for Julian date and a threshold effect for distance to forest. Antler size increased markedly up to 1500 m from forest edge and then remained stable for distances above this (table 2). The size of average antler (for a 5-year-old deer shot by a trophy stalker) decreased markedly from the start of the first hunting period; from 288.1 g on 11 May to 269.8 g on 14 June. Then, at the beginning of the next main period, antler mass declined from 276.1 g on 29 July to 257.4 g on 27 September.
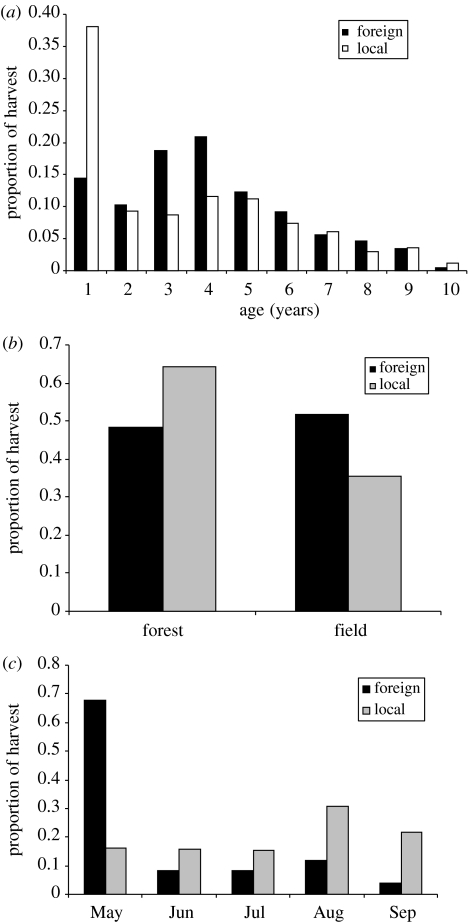
The age distribution of animals was different between foreign and local hunters (χ92=153.147, p<0.001). Local hunters shot a higher proportion of young males, but fewer prime-aged males, than foreign hunters (figure 2a). Local hunters shot a higher proportion of animals closer to the forest (figure 2b; χ12=40.307, p<0.001). Foreign hunters shot deer during different months than local hunters (χ42=461.688, p<0.001); foreign trophy stalkers harvested mainly early in the hunting season (figure 2c). This bias in time of harvest and where to shoot accounted for 41% (time 23% and space 18%) of the difference in selectivity among foreign trophy stalkers and local hunters (figure 1).
Figure 2.
Proportion of harvest by foreign trophy stalkers and local hunters in Poland differ in relation to (a) age of male roe deer, (b) distance to forest edge (forest: less than 500 m from the forest edge) and (c) month of hunting.
4. Discussion
We found that different roe deer hunters in Poland selected animals with different antler size, as would be expected from the study by Martínez et al. (2005) on red deer in Spain. In addition, we show how some of this selectivity arises, which is not necessarily due to hunting method per se, but rather we show that local hunters help to increase the chances of foreign hunters being successful, because they are paying money that also benefits non-paying local hunters. We identify two such ways: (i) foreign trophy stalkers hunt before local hunters and (ii) they hunt more often in better areas, supporting both the selectivity–depletion hypothesis and the selectivity–honey-pot hypothesis.
To be selective, there must be variation in traits among individuals, and there must be a possibility for the hunter to be selective. Martínez et al. (2005) argued that this happens due to motivation and that drive hunts give little time to select the biggest animal at the time of shooting compared to stalking, which is certainly likely. Local hunters in this Polish area use also drive hunting and often hunt with shotguns, and local hunters also avoid shooting the largest animals because greater income can be obtained by selling opportunities to take these animals to foreign hunters. The smaller size of roe deer antlers in or near forest may arise from several reasons. Firstly, habitat openness is likely positively correlated with hunter selectivity, partly since hunting close to forest is ‘sit and wait’ rather than spotting deer by car and then approaching as in open habitat. It is also likely that larger individuals live in open habitat. Higher body weight and size of field roe deer compared to forest dwelling roe deer were found in western Poland (Fruzinski et al. 1982). Even for trophy stalkers, the stock gets depleted as the season progresses, since there is competition among hunters. Having the first shot is certainly beneficial. Trophy harvesting in Spain also took place before other hunting methods, giving a potential for seasonal bias due to depletion. The age-dependent mortality induced by local and foreign hunters differed markedly. In particular, trophy stalkers shot a much lower proportion of yearlings (figure 2).
Clearly, longitudinal monitoring of individuals is the most appropriate way of getting unbiased life-history data. Such datasets are few, deriving typically from a single location, often small islands (e.g. Clutton-Brock & Coulson 2002), fenced areas (e.g. Gaillard et al. 1993) or populations being isolated by unsuitable habitat (e.g. Festa-Bianchet et al. 1998), thus representing a very biased sample of large herbivore populations. It is extremely difficult to perform longitudinal studies in harvested populations due to very short lifespan (Langvatn & Loison 1999). The data from other sources are thus required to understand the ecological change in systems, where such data cannot be collected, and hunting often provides data from vast areas and over long time frames. We concur with Martínez (2005) that knowing and possibly explicit modelling bias rather than discarding harvest data is the way forward, as such data can also have enormously important contribution in life-history studies.
Acknowledgments
We acknowledge the financial support of the Research Council of Norway to A.M. (YFF). We are grateful to W. Bresiński, R. Kamieniarz and local hunters for their help in collecting field data, and to Tim Coulson, María Martínez, Marco Festa-Bianchet and one anonymous referee for their comments.
References
- Bresinski W. Grouping tendencies in roe deer under agrocenosis conditions. Acta Theriol. 1982;27, 29:427–447. [Google Scholar]
- Clutton-Brock T.H, Coulson T. Comparative ungulate dynamics: the devil is in the detail. Phil. Trans. R. Soc. B. 2002;357:1285–1298. doi: 10.1098/rstb.2002.1128. doi:10.1098/rstb.2002.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman D.W, O'Donoghue P, Jorgenson J.T, Hogg J.T, Strobeck C, Festa-Bianchet M. Undesirable evolutionary consequences of trophy harvesting. Nature. 2003;426:655–658. doi: 10.1038/nature02177. doi:10.1038/nature02177 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Wiley; Chichester, West Sussex, UK: 2002. Statistical computing. An introduction to data analysis using S-Plus. [Google Scholar]
- Festa-Bianchet M, Gaillard J.-M, Jorgenson J.T. Mass- and density-dependent reproductive success and reproductive costs in a capital breeder. Am. Nat. 1998;152:367–379. doi: 10.1086/286175. doi:10.1086/286175 [DOI] [PubMed] [Google Scholar]
- Fruzinski B, Kaluzinski J, Baksalary J. Weight and body measurements of forest and field roe deer. Acta Theriol. 1982;27:479–488. [Google Scholar]
- Gaillard J.-M, Delorme D, Boutin J.-M, Van Laere G, Boisaubert B, Pradel R. Roe deer survival patterns: a comparative analysis of contrasting populations. J. Anim. Ecol. 1993;62:778–791. doi:10.2307/5396 [Google Scholar]
- Langvatn R, Loison A. Consequences of harvesting on age structure, sex ratio and population dynamics of red deer Cervus elaphus in central Norway. Wildl. Biol. 1999;5:213–223. [Google Scholar]
- Martínez M, Rodríquez V, Jones O.R, Coulson T, San Miguel A. Different hunting strategies select for different weights in red deer. Biol. Lett. 2005;1:353–356. doi: 10.1098/rsbl.2005.0330. doi:10.1098/rsbl.2005.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J.M, Bonenfant C, Mysterud A, Gaillard J.-M, Csányi S, Stenseth N.C. Temporal and spatial development of red deer harvesting in Europe—biological and cultural factors. J. Appl. Ecol. 2006;43:721–734. doi:10.1111/j.1365-2664.2006.01183.x [Google Scholar]
- Mysterud A, Østbye E. Comparing simple methods for ageing roe deer Capreolus capreolus: can any of them be useful in management? Wildl. Biol. 2006;12:101–107. [Google Scholar]
- Mysterud, A., Tryjanowski, P., Panek, M., Pettorelli, N. & Stenseth, N. C. Submitted. Inter-specific synchrony of two contrasting ungulates: wild boar (Sus scrofa) and roe deer (Capreolus capreolus). [DOI] [PubMed]