Abstract
Northern resident killer whales (Orcinus orca) live in highly stable groups and use group-specific vocal signals, but individual variation in calls has not been described previously. A towed beam-forming array was used to ascribe stereotyped pulsed calls with two independently modulated frequency contours to visually identified individual killer whales in Johnstone Strait, British Columbia. Overall, call similarity determined using neural networks differed significantly between different affiliation levels for both frequency components of all the call types analysed. This method distinguished calls from individuals within the same matriline better than different calls produced by a single individual and better than by chance. The calls of individuals from different matrilines were more distinctive than those within the same matriline, confirming previous studies based on group recordings. These results show that frequency contours of stereotyped calls differ among the individuals that are constantly associated with each other and use group-specific vocalizations, though across-group differences were substantially more pronounced.
Keywords: vocal variation, signature, neural network, beam-forming
1. Introduction
Variation in vocal signals often relates to the social systems in which the sounds are used (Beecher 1989), with vocal convergence occurring among affiliated group members. Greater spear-nosed bats (Phyllostomus hastatus) have group-specific screech calls. However, these are not individually distinctive and are not used by bats to distinguish among group members (Boughman & Wilkinson 1998). In other cases, signals can contain information about more than one level of social affiliation. For example, chimpanzees (Pan troglodytes verus) share some features of their pant hoot call among group members, but other features vary between individuals (Crockford et al. 2004). The individually distinctive whistles of bottlenose dolphins (Tursiops truncatus; Caldwell & Caldwell 1965) become more similar in the course of long-term associations (Watwood et al. 2004). Thus, vocal convergence at the group level does not preclude individual distinctiveness among the affiliated group members.
Fish-eating resident killer whales (Orcinus orca) in northeastern Pacific coastal waters live in highly stable family groups that produce group-specific vocalizations (Ford 1991; Riesch et al. 2006), but it is impossible to determine whether individual vocalizations vary due to the difficulty of ascribing vocalizations to particular animals. Their social structure has been well described on the basis of extensive photo-identification studies (Bigg et al. 1990); the animals live in highly stable matrilines (also referred to as matrilineal units, MU), which associate together to form pods.
Stereotyped frequency modulation patterns enable most killer whale calls to be classified into distinct types (Yurk et al. 2002), many of which contain independently modulated high- and low-frequency components (HFC and LFC; figure 1; Miller & Bain 2000; Miller 2002). These stereotyped calls are already known to vary between groups at different levels of social structure. The pods have different call repertoires and are said to belong to the same clan if two pods share any call types (Ford 1991). Slight but consistent variations in shared calls are observed both across (Ford 1987) and within the pods (Deecke 2000; Miller & Bain 2000).
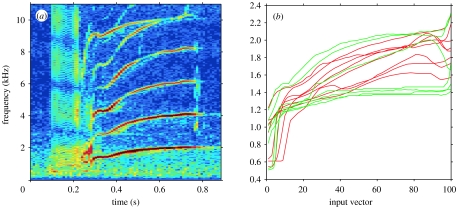
Figure 1.
(a) Spectrogram of a typical killer whale call of type N9 with two independently modulated frequency contours. (b) A sample of eight extracted LFC contours from two individuals within the same matriline, A32 (red) and A46 (green), and interpolated to an input vector of 101 points.
Here, we report on individual differences in the time–frequency contours of stereotyped calls that were ascribed to source individuals using a towed hydrophone array system (Miller & Tyack 1998). Ford (1989) proposed that killer whale group-specific vocalizations function to maintain contact among the group members. Within-group vocal exchanges with matching call types are common in these animals (Miller et al. 2004). Thus, individually distinctive acoustic features within shared call types could play a role in their social signalling. We use a neural network (NN) technique to quantify the individual distinctiveness of the time–frequency contours of two-component stereotyped calls produced by identified individuals. By comparing the individuals at two different levels of social affiliation (within and between MUs), with self comparison as a control, we describe for the first time the relative influence of group membership and individual identity on shared stereotyped acoustic signals produced by resident killer whales.
2. Material and methods
Whales were tracked from an 11 m research vessel towing a 16-element beam-forming array using focal animal sampling (Miller & Tyack 1998) in August and September 1998 and 1999 in Johnstone Strait, British Columbia. The vessel was manoeuvred to keep the focal animal separated from other whales and at a distance of approximately 100 m. The position of the focal animal relative to the vessel was recorded with a laser range finder and digital compass (Miller & Tyack 1998). The individuals were identified visually using a photo-identification catalogue, and photographs were taken to confirm identifications (Ford et al. 2000).
Recordings were processed to create spectrograms (filter bandwidth 94 Hz; dynamic range 50 dB), synchronized with directograms showing the angle of arrival of the recorded calls (Miller & Tyack 1998). The stereotyped calls were classified to call type (Ford 1987) and ascribed to focal animals separated by greater than 20° from all other animals. Frequency contours of both LFC and HFC were determined using a pitch-tracking algorithm (Wang & Seneff 2000) capable of tracking multiple harmonic structures in a signal, as the components were reasonably separated in frequency. Each contour was carefully checked for errors, and then rated on the percentage of the component that was clearly visible. Contours that were rated 100% complete were interpolated to a standard length of 100 points plus one value for call duration, making an input vector of 101 points (Deecke et al. 1999; figure 1).
We used a multi-layer feed-forward network with supervised back-propagation learning (Deecke et al. 1999) to compare contours of the same call type and component (LFC only, HFC only and LFC–HFC combined sequentially for an input vector of 201 points). Eight calls were randomly selected from all those available (for a call type/component) from each of the two individuals. A ‘jacknifing’ classification was performed on that set of 16 calls; one call was sequentially removed, the NN was trained on the remaining 15, and the jacknifed call was classified to one of the two individuals by the network. Average discrimination error (ADE) was calculated as the proportion of incorrectly classified calls, averaged over 30 randomly selected sets of 16 calls and grouped into a social-affiliation type. For each component, call type and social affiliation, ADE was averaged across all the individuals. ADE is lower when calls are more distinctive, with the chance value at 50%.
Our data enabled comparisons at two levels of social affiliation between the two individuals; those from different MUs (between-MU comparisons) and those from the same MU (within-MU comparisons; table 1). Different calls from the same individual (self comparisons) were used as a control. The significance levels of ADEs for social-comparison type, call type and contour input were calculated using a three-way analysis of variance and post hoc Tukey tests (Zar 1996).
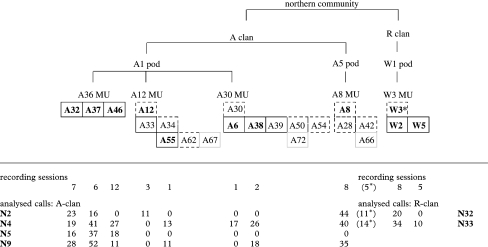
Table 1.
Group membership of analysed individuals (bold text) and their direct relatives, defined by sex: males (solid boxes) females (dashed boxes) and juveniles (grey boxes). (Sample size of calls and recording sessions are listed below each individual.)
3. Results
A total of 72 separate recording sessions of northern resident killer whales were conducted, yielding 1508 vocalizations produced by 19 whales in A and R clans. Based on 58 recording sessions, 11 individual whales from five different MUs, three pods and two clans had enough calls to be analysed (table 1). A total of 592 calls of six different call types were used to make 189 comparisons: 65 LFC, 62 HFC and 62 LFC–HFC combined comparisons.
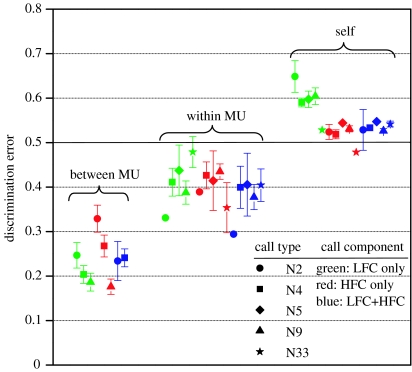
Calls from the same individual had the highest ADE (LFC, 0.60±0.04; HFC, 0.52±0.02; combined, 0.53±0.02; figure 2). Calls from two members of the same MU had ADE slightly better than chance (LFC, 0.42±0.07; HFC, 0.41±0.07; combined, 0.39±0.08). Finally, calls from two members of different MUs had the lowest ADE (LFC, 0.20±0.08; HFC, 0.24±0.10; combined, 0.21±0.09). The difference in ADE between levels of social affiliation was statistically significant (three-way ANOVA: F2,150=50.73, p<0.001), while neither component nor call type had a significant effect on discrimination error (ANOVA; component: F2,150=0.370, p=0.69; call type: F3,150=0.99, p=0.40). Post hoc Tukey comparisons showed significant differences between all social-levels for all three contour analyses.
Figure 2.
Average discrimination error (±95% CI) as measured by pairwise NN comparison with chance discrimination at 0.5 (solid line). Points show ADE of each component/call type combination, grouped by social-comparison type (brackets). ADE differed significantly by social-comparison for all the three components analysed, with less difference by call type.
4. Discussion
The ability of the NN to classify shared calls to one of the two individuals depended primarily on their degree of social affiliation, with less variation among call types or low- or high-frequency contours (figure 2). Calls produced by the same individual, used as a control, were classified slightly worse than chance. This could simply be because jacknifing left only seven calls in the ‘correct’ group as opposed to eight in the other, and NNs can be sensitive to training set sample size (Deecke et al. 1999). Nonetheless, the NN was able to discriminate between the randomly selected time–frequency contours of different individuals within the same matrilineal group better than chance (and better than the self-comparison control), providing the first indication that shared killer whale calls contain some degree of individual signature information. Calls of individuals from different matrilines were much more strongly distinguishable, confirming previous studies that reported between-MU differences based on group recordings, where individual signallers were not identified (Deecke et al. 1999; Miller & Bain 2000). The time–frequency contours of stereotyped calls seem to be primarily shaped by group-specific convergence rather than individual distinctiveness.
This study has shown that variations within shared call types represent a hierarchical system, with strongly recognizable group signatures and less prominent individual signatures, which may allow both efficient transmission of group-level information and individual identity discrimination. Many behaviours, such as direction changes or cooperative foraging, may involve all the group members. In such contexts, a group-specific call may enable each group member to extract the relevant information from the background calls from other groups. Less-distinctive cues may suffice for individual recognition, as a receiver has only to discriminate among a few group members (Beecher 1989). Other call features, besides the contours considered here, could also facilitate individual identification, such as differences in the distribution of energy across harmonics.
The call types analysed here are the most intense produced by resident killer whales and tend to occur when they are more spaced (Miller 2006). Less-intense sound types (generally without an HFC), whistles (Riesch et al. 2005) and variable calls appear to be more common when animals are closer together and between-group dynamics, are potentially less important. The investigation of individual variation in these sounds would be interesting, but our array technique cannot reliably ascribe sounds to closely spaced signallers. Signatures at different social levels must ultimately be tested using playback experiments to determine whether killer whales themselves perceive and respond to the reported differences. However, the presence of individual signatures strong enough to be distinguished with statistical analysis suggests the potential for individuals to acoustically distinguish between the highly similar shared calls of their matrilineal relatives.
Acknowledgments
Thanks to many field assistants, those who built the sound corpus at MIT, and Volker Deecke. Funding was provided by WHOI's Coastal Research Center and Ocean Ventures Fund, and a Royal Society USA/Canada fellowship to P.J.O.M. All the field recordings were made under a Canadian permit, following Canadian Law.
References
- Beecher M.D. Signalling systems for individual recognition: an information theory approach. Anim. Behav. 1989;38:248–261. [Google Scholar]
- Bigg M.A, Olesiuk P.K, Ellis G, Ford J.K.B, Balcomb K.C. Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Comm. (special issue) 1990;12:383–406. [Google Scholar]
- Boughman J.W, Wilkinson G.S. Greater spear-nosed bats discriminate group mates by vocalizations. Anim. Behav. 1998;55:1717–1732. doi: 10.1006/anbe.1997.0721. doi:10.1006/anbe.1997.0721 [DOI] [PubMed] [Google Scholar]
- Caldwell M.C, Caldwell D.K. Individualized whistle contours in bottlenosed dolphins, Tursiops truncatus. Nature. 1965;207:434–435. doi:10.1038/207434a0 [Google Scholar]
- Crockford C, Herbinger I, Vigilant L, Boesch C. Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology. 2004;110:221–243. doi:10.1111/j.1439-0310.2004.00968.x [Google Scholar]
- Deecke V.B, Ford J.K.B, Spong P. Quantifying complex patterns of bioacoustic variation: Use of a neural network to compare killer whale (Orcinus orca) dialects. J. Acoust. Soc. Am. 1999;105:2499–2507. doi: 10.1121/1.426853. doi:10.1121/1.426853 [DOI] [PubMed] [Google Scholar]
- Ford J.K.B. A catalogue of underwater calls produced by killer whales (Orcinus orca) in British Columbia. Can. Data Rep. Fish. Aquat. Sci. 1987;633:1–165. [Google Scholar]
- Ford J.K.B. Acoustic behavior of resident killer whales (Orcinus orca) in British Columbia. Can. J. Zool. 1989;67:727–745. [Google Scholar]
- Ford J.K.B. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can. J. Zool. 1991;69:1454–1483. [Google Scholar]
- Ford J.K.B, Ellis G.M, Balcomb K.C. UBC Press; Vancouver, WA: 2000. Killer whales; the natural history and genealogy of Orcinus orca in British Columbia and Washington State. [Google Scholar]
- Miller P.J.O. Mixed-directionality of killer whale stereotyped calls: a direction of movement cue? Behav. Ecol. Sociobiol. 2002;52:262–270. doi:10.1007/s00265-002-0508-9 [Google Scholar]
- Miller P.J.O. Diversity in the sound pressure levels and estimated active space of resident killer whale vocalizations. J. Comp. Phys. A. 2006;192:449–459. doi: 10.1007/s00359-005-0085-2. doi:10.1007/s00359-005-0085-2 [DOI] [PubMed] [Google Scholar]
- Miller P.J.O, Bain D.E. Within-pod variation in the sound production of a pod of killer whales, Orcinus orca. Anim. Behav. 2000;60:617–628. doi: 10.1006/anbe.2000.1503. doi:10.1006/anbe.2000.1503 [DOI] [PubMed] [Google Scholar]
- Miller P.J.O, Tyack P.L. A small towed beamforming array to identify vocalizing resident killer whales (Orcinus orca) concurrent with focal behavioral observations. Deep Sea Res. II. 1998;45:1389–1405. doi:10.1016/S0967-0645(98)00028-9 [Google Scholar]
- Miller P.J.O, Shapiro A.D, Tyack P.L, Solow A.R. Call-type matching in vocal exchanges of free-ranging resident killer whales, Orcinus orca. Anim. Behav. 2004;67:1099–1107. doi:10.1016/j.anbehav.2003.06.017 [Google Scholar]
- Riesch R, Ford J.K.B, Thomsen F. Stability and group specificity of stereotyped whistles in resident killer whales, Orcinus orca, off British Columbia. Anim. Behav. 2005;71:79–91. doi:10.1016/j.anbehav.2005.03.026 [Google Scholar]
- Wang, C. & Seneff, S. 2000 Robust pitch tracking for prosodic modeling in telephone speech. In IEEE Int. Conf. on Acoustics, Speech and Signal Processing, Istanbul, Turkey.
- Watwood S.L, Tyack P.L, Wells R.S. Whistle sharing in paired male bottlenose dolphins, Tursiops truncatus. Behav. Ecol. Sociobiol. 2004;55:531–543. doi:10.1007/s00265-003-0724-y [Google Scholar]
- Yurk H, Barrett-Lennard L, Ford J.K.B, Matkin C.O. Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Anim. Behav. 2002;63:1103–1119. doi:10.1006/anbe.2002.3012 [Google Scholar]
- Zar J.H.Biostatistical analysis1996Prentice-Hall; Upper Saddle River, NJ [Google Scholar]