Abstract
Olfactory learning in insects is a useful model for studying neural mechanisms underlying learning and memory, but memory storage capacity for olfactory learning in insects has not been studied. We investigate whether crickets are capable of simultaneously memorizing seven odour pairs. Fourteen odours were grouped into seven A/B pairs, and crickets in one group were trained to associate A odours with water reward and B odours with saline punishment for all the seven pairs. Crickets in another group were trained with the opposite stimulus arrangement. Crickets in all the groups exhibited significantly greater preference for the odours associated with water reward for all the seven odour pairs. We conclude that crickets are capable of memorizing seven odour pairs at the same time.
Keywords: olfactory learning, memory capacity, cricket, conditioning
1. Introduction
The ability to memorize a number of odours is important for animals, especially for those that rely on olfaction for finding foods, such as rodents and many species of insects. Experiments with rats using two-odour go–no go discrimination tasks have demonstrated that they are capable of memorizing 16 (Lovelace & Slotnick 1995; Slotnick 2001) or 30 (Staubli et al. 1987) pairs of odours at the same time, with no sign of limitation of memory capacity. Many insects, including honeybees (Apis mellifera), fruit-flies (Drosophila melanogaster) and cockroaches (Periplaneta americana), have excellent olfactory learning (Menzel 1999; Heisenberg 2003; Watanabe & Mizunami 2006), and they are used as models to study olfactory learning and memory and their underlying neural mechanisms (Menzel 1999; Heisenberg 2003; Unoki et al. 2005; Matsumoto et al. 2006). However, as far as we know, no attempt has been made to examine the memory storage capacity, which is a fundamental feature used to characterize a memory system, in the learning of olfactory or other sensory signals in insects, except Reinhard et al. (2006) who demonstrated that honeybees are able to associate two different odours with two different feeding locations.
We have shown that crickets, Gryllus bimaculatus, have excellent capability of olfactory learning, characterized by fast acquisition, long retention and easy re-writing of memory (Matsumoto & Mizunami 2002; Matsumoto et al. 2006). In this study, we examined whether crickets are capable of memorizing seven pairs of odours at the same time, using a classical conditioning and an operant testing procedure described previously (Matsumoto & Mizunami 2002).
2. Material and methods
Adult male crickets, G. bimaculatus reared in a 12 h light–dark cycle at 27±2°C, were used for the experiments. Four days before the start of the experiment, crickets were placed in a container and fed a diet of insect pellets ad libitum, but they were deprived of drinking water to enhance their motivation to search for water. On the day of the experiment, they were individually placed in 100 ml glass beakers.
The conditioning procedure has been described (Matsumoto & Mizunami 2002); briefly, individual crickets were given differential conditioning trials to associate one odour with reward and another odour with punishment using 1 ml hypodermic syringes. A small filter paper attached to the needle of the syringe at 10 mm from its tip was soaked with odorant solution. The syringe used for appetitive or aversive conditioning was filled with water or 20% NaCl solution, respectively. For conditioning, the filter paper was placed within 1 cm of the cricket's head, and 2 s later, a drop of water or saline was passed to the mouth of the cricket.
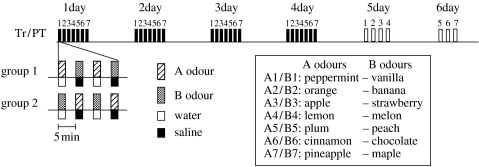
Figure 1 shows the time schedule of conditioning and odour preference tests. Fourteen different odorants (natural essences) were arbitrarily grouped into seven A/B odour pairs (figure 1, inset). The crickets were divided into two groups and they received two sets of differential conditioning trials with opposite stimulus arrangement: crickets in group 1 received conditioning trials to associate A odours with water reward and B odours with saline and those in group 2 received conditioning trials to associate B odours with water and A odours with saline for seven A/B odour pairs. The intervals between trials were 5 min. The conditioning trials for each of the seven odour pairs were performed at intervals of 90 min. The training was repeated for 4 consecutive days. After the cessation of training, each cricket was given a diet of insect pellets ad libitum in a beaker until it was subjected to an odour preference test.
Figure 1.
The time schedule for training (Tr, black bars) and preference test (PT, white bars). Crickets were kept in 12 h light–dark cycle and they received training and preference tests in the photophase. The training session consists of two differential conditioning trials to associate A odour (hatched bar) with water (white square) and B odour (shaded bar) with saline (black square) (for group 1 crickets), or to associate B odour with water and A odour with saline (for group 2 crickets). The intervals between trials were 5 min. The conditioning trials were performed for seven odour pairs at intervals of 90 min (Tr-1–7). Training was performed on 4 consecutive days. One day after the cessation of 4 day training, odour preference was tested for A1/B1, A2/B2, A3/B3 and A4/B4 pairs (PT-1–4). On the next day, preference was tested for the remaining A5/B5, A6/B6 and A7/B7 pairs (PT-5–7). Sources of odorants (inset) were: Miyako Kosho (Tokyo, Japan) for peppermint essence; Kyoritsu Shokuhin (Tokyo) for vanilla essence; Narizuka (Tokyo) for banana, orange, melon and maple essences; Meijiya (Tokyo) for strawberry and lemon essences and Asaoka (Tokyo) for all other essences.
One day after the cessation of 4-day training, relative odour preference was tested for each of the four odour pairs (A1/B1–A4/B4) at intervals of 2 h. On the next day, preference tests for the remaining three odour pairs (A5/B5–A7/B7) were performed at intervals of 2 h. The procedure for odour preference test has been described in detail in Matsumoto & Mizunami (2002). On the floor of test chamber, there were two circular holes that connected the chamber with two odour sources. Each odour source consisted of a cylindrical plastic container containing a filter paper soaked with odorant solution covered with gauze net. Each cricket was allowed to visit odour sources for a 4 min test period, and the time the cricket spent at each source, i.e. the time that it probed the net top with its mouth, was measured cumulatively.
Relative odour preference of crickets in a given test was statistically evaluated by comparing the time each cricket spent at the rewarded odour source with that spent at the negatively reinforced odour source with Wilcoxon's test (WCX test).
3. Results
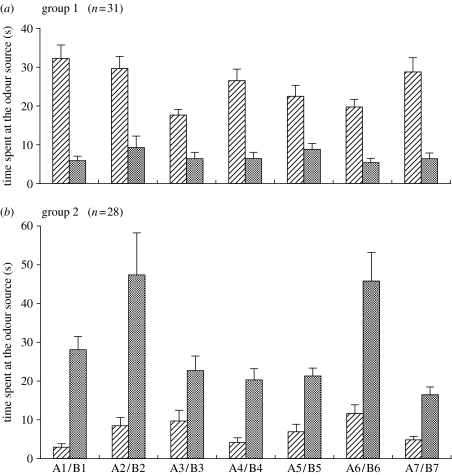
Figure 2a shows the relative odour preferences of group 1 crickets that received conditioning trials to associate A odours with water reward and B odours with saline punishment for seven odour pairs. They spent significantly more time at the positively reinforced odour source than at the negatively reinforced odour source for all the seven odour pairs (T=0.0 in A1/B1 pair; T=28.5 in A2/B2 pair; T=45.0 in A3/B3 pair; T=0.0 in A4/B4 pair; T=50.5 in A5/B5 pair; T=2.0 in A6/B6 pair; T=1.0 in A7/B7 pair; p<0.001 for all the odour pairs, n=31, WCX test).
Figure 2.
Odour preferences of (a) group 1 crickets (n=31) that received conditioning trials to associate A odours with water and B odours with saline and (b) those of group 2 crickets (n=28) that received conditioning trials to associate B odours with water and A odours with saline for seven A/B odour pairs. The time spent at the source of A odour (hatched bars) and that spent at the source of B odour (shaded bars) for each of the seven odour pairs are shown as means+s.e. These were significantly different (p<0.001, WCX test) for all the seven pairs in both groups.
Figure 2b shows relative odour preferences of group 2 crickets that received conditioning trials to associate B odours with water reward and A odours with saline punishment. They also spent significantly more time at the rewarded odour source than at the negatively reinforced odour source for all the seven odour pairs (T=0.0 in A1/B1 pair; T=0.0 in A2/B2 pair; T=24.0 in A3/B3 pair; T=11.0 in A4/B4 pair; T=24.0 in A5/B5 pair; T=3.0 in A6/B6 pair; T=0.0 in A7/B7 pair; p<0.001 for all the odour pairs, n=28, WCX test).
The results indicate that crickets are capable of memorizing seven pairs of odours at the same time.
4. Discussion
Olfactory learning in insects has proved to be a pertinent model for studying many aspects of learning and memory and their neural mechanisms (Menzel 1999; Heisenberg 2003). However, as far as we know, olfactory memory storage capacity, or memory storage capacity for other sensory signals, has not been studied in insects, except that Reinhard et al. (2006) have shown that honeybees are able to memorize two odours in association with two feeding locations. In this study, we found that crickets are capable of selecting a reward-associated odour from a pair of odours for seven odour pairs at the same time. Since this learning was achieved by a relatively small number of conditioning trials, i.e. only eight conditioning trials for each odour, it is likely that olfactory memory capacity of crickets is still far from saturation.
The studies in rodents using a two-odour discrimination task have demonstrated that rats are capable of memorizing 16 (Lovelace & Slotnick 1995) or 30 (Staubli et al. 1987) pairs of odours at the same time. It is argued that the rats may be equipped with a ‘data’ memory system to deal with a practically unlimited volume of olfactory memory, as are humans (Staubli et al. 1987). It would be interesting to further examine to what extent olfactory memory capacity of insects approaches that of rodents.
Our finding that crickets possess a much higher storage capacity for odour memory than that known previously provides a hint that the basic organization of the neural system in processing and storing olfactory memory should be reconsidered. The studies in honeybees suggest that the antennal lobe (the primary olfactory centre) and the mushroom body (higher olfactory centre as well as multisensory centre) participate in olfactory learning (Menzel 1999). The mushroom body of crickets consists of an unusually large number (50 000) of intrinsic neurons (Schürmann 1973), and thus, may be suited to processing and storing a large volume of odour memory. In contrast, the antennal lobe consists of a small number (approx. 1000) of neurons and may be less suited to processing and storing a large volume of olfactory memory, although this number of neurons may be sufficient for memorizing seven pairs of odours at the same time. Future study on the limitation of olfactory memory capacity of crickets may help to further elucidate the basic organizations of the neural system for olfactory memory storage.
The high capacity of olfactory memory storage in crickets found in this study may reflect their omnivorous feeding habit, as has been argued for rodents (Slotnick 2001). Crickets usually feed on vegetables, fruits, fallen leaves and carcasses of small animals. Since the availability of their food items varies with seasons, they may test many potential food items to see whether they are edible or not. Therefore, a large capacity for odour memory may play a critical role in appropriate food selection, especially for nocturnal animals like crickets. Crickets are known to use cuticular contact pheromone for mating (Tregenza & Wedell 1997), and it would be interesting to see whether crickets are capable of memorizing contract pheromone of individuals for mate choice.
A correlation between memory storage capacity and ecological requirements has been discussed for birds. Male song sparrows (Melospiza melodia), territorial songbirds that need to memorize many kinds of songs of conspecific males to defend territory, could learn up to 32 pairs of songs (Stoddard et al. 1992), while Bengalese finches (Lonchura domestica), non-territorial songbirds that do not need to learn songs of conspecific males, learned only two to six pairs of songs, and the brain area possibly involved in the processing of song memory has been discussed (Ikebuchi & Okanoya 2000). In food-storing birds that have vast capacity of visual spatial memory, it has been argued that the development of spatial memory is correlated with the increased size of the hippocampus (Sherry et al. 1992). Comparison of olfactory memory capacities in different species of insects with different feeding habits, as well as comparison of the development of olfactory centres in the brain, would be an interesting subject for the future study.
Acknowledgments
This research was funded by grants from the Ministry of Education, Science, Culture, Sports and Technology of Japan.
References
- Heisenberg M. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 2003;4:266–274. doi: 10.1038/nrn1074. doi:10.1038/nrn1074 [DOI] [PubMed] [Google Scholar]
- Ikebuchi M, Okanoya K. Limited auditory memory for conspecific songs in a non-territorial songbird. NeuroReport. 2000;11:3915–3919. doi: 10.1097/00001756-200011270-00061. [DOI] [PubMed] [Google Scholar]
- Lovelace C.T, Slotnick B.M. Memory for brief, widely spaced odor presentations in the rat. Chem. Senses. 1995;20:183–190. doi: 10.1093/chemse/20.2.183. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Mizunami M. Temporal determinants of olfactory long-term retention in the cricket Gryllus bimaculatus. J. Exp. Biol. 2002;205:1429–1437. doi: 10.1242/jeb.205.10.1429. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Unoki S, Aonuma H, Mizunami M. Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory. Learn. Mem. 2006;13:35–44. doi: 10.1101/lm.130506. doi:10.1101/lm.130506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. A. 1999;185:323–340. doi:10.1007/s003590050392 [Google Scholar]
- Reinhard J, Srinivasan M.V, Zhang S. Complex learning in honeybees: can there be more than two? J. Comp. Physiol. A. 2006;192:409–416. doi: 10.1007/s00359-005-0079-0. doi:10.1007/s00359-005-0079-0 [DOI] [PubMed] [Google Scholar]
- Schürmann F.W. Über die Struktur der Pilzkörper des Insektenhirns. III. Die Anatomie der Nervenfasern in den Corpora pedunculata bei Acheta domesticus L. (Orthoptera): eine Golgi-Studie. Z. Zellforsch. 1973;145:247–285. doi:10.1007/BF00307391 [PubMed] [Google Scholar]
- Sherry D.F, Jacobs L.F, Gaulin S.J.C. Spatial memory and adaptive specialization of the hippocampus. Trends Neurosci. 1992;15:298–303. doi: 10.1016/0166-2236(92)90080-r. doi:10.1016/0166-2236(92)90080-R [DOI] [PubMed] [Google Scholar]
- Slotnick B.M. Animal cognition and the rat olfactory system. Trends Cogn. Sci. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. doi:10.1016/S1364-6613(00)01625-9 [DOI] [PubMed] [Google Scholar]
- Staubli U, Fraser D, Faraday R, Lynch G. Olfaction and the “data” memory system in rats. Behav. Neurosci. 1987;101:757–765. doi: 10.1037//0735-7044.101.6.757. doi:10.1037/0735-7044.101.6.757 [DOI] [PubMed] [Google Scholar]
- Stoddard P.K, Beecher M.D, Loesche P, Campbell S.E. Memory does not constrain individual recognition in a bird with song repertoires. Behaviour. 1992;122:274–287. [Google Scholar]
- Tregenza T, Wedell N. Definitive evidence for cuticular pheromones in a cricket. Anim. Behav. 1997;54:979–984. doi: 10.1006/anbe.1997.0500. doi:10.1006/anbe.1997.0500 [DOI] [PubMed] [Google Scholar]
- Unoki S, Matsumoto Y, Mizunami M. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 2005;22:1409–1416. doi: 10.1111/j.1460-9568.2005.04318.x. doi:10.1111/j.1460-9568.2005.04318.x [DOI] [PubMed] [Google Scholar]
- Watanabe H, Mizunami M. Classical conditioning of activities of salivary neurons in an insect. J. Exp. Biol. 2006;209:766–779. doi: 10.1242/jeb.02049. doi:10.1242/jeb.02049 [DOI] [PubMed] [Google Scholar]