Abstract
We studied the effects of fungal endophyte infection of meadow ryegrass (Lolium pratense=Festuca pratensis) on the frequency of the barley yellow dwarf virus (BYDV). The virus is transferred by aphids, which may be deterred by endophyte-origin alkaloids within the plant. In our experiment, we released viruliferous aphid vectors on endophyte-infected and endophyte-free plants in a common garden. The number of aphids and the percentage of BYDV infections were lower in endophyte-infected plants compared to endophyte-free plants, indicating that endophyte infection may protect meadow ryegrass from BYDV infections.
Keywords: endophytes, grasses, barley yellow dwarf virus, aphids, multitrophic interactions
1. Introduction
Systemic fungal endophytes live inside many Pooidae grasses. These fungi are asymptomatic and may form a mutualistic relationship with their hosts (Clay 1988; Saikkonen et al. 2004; Schardl et al. 2004), increasing the host's growth (Arachevaleta et al. 1989), herbivore resistance (Cheplick & Clay 1988; Breen 1994) and tolerance to various stresses (Arachevaleta et al. 1989; Bacon 1993). In turn, the endophyte receives nutrients, carbohydrates and shelter from its host. Recently, it has been acknowledged that the nature of the relationship varies from mutualistic to antagonistic (Saikkonen et al. 1998; Faeth 2002), depending on, for example, environmental conditions (Cheplick et al. 1989; Lehtonen et al. 2005a), the genetic background of the grass and fungus (Faeth et al. 2002) and interacting species in the community (Saikkonen et al. 2004; Lehtonen et al. 2005b).
Experimental research has mostly focused on direct, pairwise interactions between host plant and fungal endophytic symbiont, or tripartite interactions, including herbivores (e.g. Clay 1988; Bacon 1993; Breen 1994; Saikkonen et al. 1998). Species interactions in natural systems, however, are likely to be more complex. For example, plant pathogens may be affected by endophytic fungi, but only a limited number of studies have investigated pathogen–endophyte interactions (Schardl et al. 2004). Endophytes have been hypothesized to inhibit plant pathogens and thus benefit their host (Clay 1988). Endophytes have been observed to reduce pathogenic growth in cultures (Siegel & Latch 1991; Yue et al. 2000) and in planta (Stovall & Clay 1991; Gwinn & Gavin 1992), but contradictory or neutral results have also been obtained (Burpee & Bouton 1993; Hamilton & Faeth 2005). The mechanism of increased disease resistance in endophyte-infected plants is largely unknown, but it is hypothesized that compounds produced by endophytes may at least play a partial role in this phenomenon (Yue et al. 2000).
A special case of plant pathogens is the plant viruses, because the properties of the host plant can affect them both directly by host metabolites and indirectly via effects of plant quality on insect vectors transmitting the viruses from host to host. Barley yellow dwarf virus (BYDV) is one of the most harmful cereal viruses, and it is transmitted to cereals from perennial grasses near the crop field by aphid vectors (Rochow & Duffus 1981). The aphids get the virus by feeding on contaminated plants. The aphids carry the virus, but do not transmit the infection to their offspring. BYDV is transmitted by aphids which could be deterred by endophyte-origin alkaloids (Schardl & Phillips 1997). Thus, it is possible that endophyte infection influences virus transmission. However, the results obtained from the few endophyte–BYDV studies have been contradictory, e.g. Guy (1992) found no correlation between virus infection and the incidence of endophyte in perennial ryegrass (Lolium perenne), whereas other correlative studies have revealed that some endophyte-infected tall fescue (Festuca arundinaceum) origins seem to be more resistant to BYDV than the others (Mahmood et al. 1993; Guy & Davis 2002). In this common garden experiment, our aim was to examine how endophyte infection affects BYDV transmission in meadow ryegrass (Lolium pratense).
2. Material and methods
Meadow ryegrass is commonly infected by systemic and strictly vertically transmitted fungal endophyte Neotyphodium uncinatum (Craven et al. 2001). Among Nordic cultivars of meadow ryegrass, endophyte frequencies vary from 0 to 100% (Saikkonen et al. 2000), and there are notable differences also within cultivars among seed lots (Lehtonen et al., unpublished data).
The bird cherry oat aphid (Rhopalosiphum padi) was used as a vector and as a herbivore in this study, because it is a natural herbivore of meadow ryegrass (Heie 1981) and an important transmitter of BYDV (Guy et al. 1987). More specifically, R. padi is the transmitter of the PAV strain of BYDV, which is the most commonly occurring and the most severe of the different BYDVs worldwide (Wang et al. 2000) and in the Nordic countries, including Finland and Baltic countries (Bisnieks et al. 2004).
Mature meadow ryegrass (L. pratense) individuals (diameter approx. 10 cm, consisting of ca 30 tillers) were randomly chosen from an old pasture located in southwestern Finland (Lehtonen et al. 2005a) and transplanted randomly into a common garden near to the pasture into four blocks, seven endophyte-infected (E+) and seven uninfected (E−) plants in each block in August 2001. The grass samples to control for the natural infections were taken before the beginning of the experiment. Aphids were fed on BYDV-PAV-infected oat leaves for one week before the experiment. Twenty-five aphids carrying BYDV-PAV were released into each block and were left to reproduce for two months. The blocks were covered with organdy cages (1.5 m×1.5 m×1 m (height)) to prevent viral infections from spreading to the neighbouring plants. The soil was nutrient-rich and high in organic matter (manured the previous spring). The plants were harvested in October 2001, aphids were counted and samples for the BYDV-PAV analysis were taken. Tillers were dried and weighed.
The statistical analyses were performed with the SAS statistical package (v. 8.02), with the GENMOD procedure. Logistic regression for the occurrence of BYDV-PAV infection was calculated with binomial distribution and logit link, with endophyte infection and block as independent factors. General linear models for the numbers of aphids and plant biomasses were calculated separately with normal distribution and identity link, again with endophyte infection and block as independent factors. The numbers of aphids and plant biomasses were logarithm transformed to fit the requirements of the models. p-values are based on type 3 chi-square values in all the analyses (SAS Technical Report P-243 1993).
Leaf samples, two to four leaves (about 0.5 g), were taken for BYDV-PAV analysis before and after the aphid experiment. Leaves were frozen at −20 °C for one to three months until assayed by ELISA. Approximately 0.1 g of each sample was ground in 1 ml of the sample extraction buffer (specified by Bioreba Ag., Reinach, Germany) and detected by the DAS-ELISA assay. BYDV-PAV-specific antisera, conjugate and the alkaline phosphate substrate (Bioreba) were used according to the manufacturer's instructions. After 1 hour of incubation from substrate addition, the absorbances were measured at the optical density of 620 nm. Absorbance values at least twice as high as in healthy oat leaves (comparable to healthy meadow ryegrass leaves) were considered positive for BYDV-PAV infection
3. Results
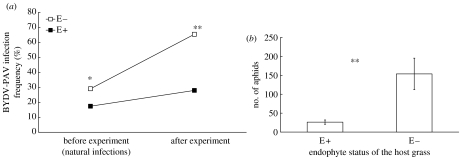
We found BYDV-PAV to infect E+ plants less frequently than E− plants (figure 1a). Since some of the plants were infected before the beginning of the experiment, we analysed the BYDV-PAV infections separately for plants that were infected before the experiment and excluded these plants from the final analysis (number of replicates for the final analysis: E+, 22; E−, 19). The results of both analyses showed significant effects of endophyte infection in reducing infection frequency by BYDV-PAV (table 1). The infection frequencies before and after the experiment are shown in figure 1a. The effects of block and the block×endophyte interaction on BYDV-PAV infections were non-significant in both analyses (table 1). Endophyte infection reduced the number of aphids (figure 1b; table 1). There were no differences in biomasses between endophyte-infected and uninfected plants (table 1).
Figure 1.
(a) Effects of endophyte infection on BYDV-PAV infection frequency (%) before the experiment (plants naturally BYDV-infected in the field before transplantation) and after the experiment in the common garden. (b) Number of aphids on endophyte-infected (E+) and endophyte-free plants (E−) (mean±s.e.). *p≤0.05, **p≤0.01.
Table 1.
Results of logistic regressions and general linear models by GENMOD showing the effects of endophyte infection and block on BYDV-PAV infections before and after the experiment, number of aphids and plant biomass. (Significant p-values (p<0.05) are shown in bold.)
| BYDV (before the experiment) | BYDV (after the experiment) | number of aphids | plant biomass | ||||||
|---|---|---|---|---|---|---|---|---|---|
| source of variation | d.f. | χ2 | p | χ2 | p | χ2 | p | χ2 | p |
| endophyte | 1 | 3.74 | 0.05 | 5.08 | 0.02 | 8.79 | 0.003 | 1.28 | 0.26 |
| block | 3 | 4.79 | 0.19 | 3.25 | 0.35 | 29.52 | <0.0001 | 2.62 | 0.46 |
| endophyte×block | 3 | 4.07 | 0.25 | 3.60 | 0.31 | 2.25 | 0.52 | 1.98 | 0.68 |
4. Discussion
We found endophyte infection to lower the frequencies of BYDV in meadow ryegrass. Endophyte-infected meadow ryegrass plants harboured less viral infections both in natural and common garden conditions than uninfected plants. The reproduction of bird cherry oat aphids was decreased on endophyte-infected plants compared to uninfected plants. We assume that the poor performance of aphids on E+ plants is the main reason for the lower BYDV-PAV infection frequency in endophyte-infected meadow ryegrass. In a previous study, we found bird cherry oat aphid to be deterred by endophyte-infected meadow ryegrass in greenhouse conditions, especially at high soil nutrient levels (Lehtonen et al. 2005a). The alkaloids produced by the Neotyphodium-infected meadow ryegrass were identified to be lolines (Lehtonen et al. 2005b), which are known to deter a wide range of insect herbivores (Schardl & Phillips 1997). The effects of endophytes on BYDV frequencies are likely to depend on alkaloid types produced by the fungus–plant symbiosis, because the susceptibility of aphid species to different alkaloids greatly varies (Siegel 1990; Eichenseer & Dahlman 1992). Yet, we cannot exclude the possible role of a biochemical factor in endophyte–BYDV interactions. For example, some alkaloids are shown to have antiviral activities (e.g. Wang et al. 2004), though they have not been reported in alkaloid-producing grass–endophyte systems. Few studies examining effects of endophytes on BYDV transmission in grasses exist—and they are of correlative nature—have shown inconsistent results in terms of BYDV frequencies in endophyte-infected plants (Guy 1992; Mahmood et al. 1993; Guy & Davis 2002).
The effects of BYDV infection on the performance of pasture grasses are variable (Catherall & Parry 1987; Clarke & Eagling 1994). The alkaloid production of E+ perennial ryegrass was not affected by BYDV infection and there were no differences in herbage yield between BYDV-infected E+ and E− plants, although there were genotype-related differences (Hesse & Latch 1999). However, even if the BYDV may infect (E+ and E−) grasses without causing any direct loss for their fitness, its presence in the grass may serve as a reservoir for subsequent infection of other agricultural crops. Thus, low infection rate of BYDV in E+ meadow ryegrass may protect the adjacent plants from BYDV infections. This phenomenon may be used in agricultural practises by sowing E+ meadow ryegrass next to cereals that may suffer heavily for BYDV infection, and thereby trying to reduce yield losses caused by the virus.
Acknowledgements
We thank Kalle Lehtonen for providing the pastures and fields for experimental utilization and helping on the field, and two anonymous referees for helpful comments on the manuscript. Daniel Rego kindly checked the language. This study was financially supported by the Academy of Finland, the Finnish Cultural Foundation and the Turku University Foundation.
References
- Arachevaleta M, Bacon C.W, Hoveland C.S, Radcliffe D.E. Effect of the tall fescue endophyte on plant response to environmental stress. Agron. J. 1989;81:83–90. [Google Scholar]
- Bacon C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993;44:123–141. doi:10.1016/0167-8809(93)90042-N [Google Scholar]
- Bisnieks M, Kvarnheden A, Sigvald R, Valkonen J.P.T. Molecular diversity of the coat protein-encoding region of barley yellow dwarf virus-PAV and barley yellow dwarf virus-MAV from Latvia and Sweden. Arch. Virol. 2004;149:843–853. doi: 10.1007/s00705-003-0242-2. doi:10.1007/s00705-003-0242-2 [DOI] [PubMed] [Google Scholar]
- Breen J.P. Acremonium endophyte interactions with enhanced plant resistance to insects. Annu. Rev. Entomol. 1994;39:401–423. doi:10.1146/annurev.en.39.010194.002153 [Google Scholar]
- Burpee L.L, Bouton J.H. Effect of eradication of the endophyte Acremonium coenophialum on epidemics of Rhizoctonia blight in tall fescue. Plant Dis. 1993;77:157–159. [Google Scholar]
- Catherall P.L, Parry A.L. Effects of barley yellow dwarf virus on some varieties of Italian, hybrid and perennial ryegrasses and their implication for grass breeders. Plant Pathol. 1987;36:148–153. [Google Scholar]
- Cheplick G.P, Clay K. Acquired chemical defenses in grasses—the role of fungal endophytes. Oikos. 1988;52:309–318. [Google Scholar]
- Cheplick G.P, Clay K, Marks S. Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol. 1989;111:89–97. doi:10.1111/j.1469-8137.1989.tb04222.x [Google Scholar]
- Clarke R.G, Eagling D.R. Effects of pathogens on perennial pasture grasses. N. Z. J. Agric. Res. 1994;37:319–327. [Google Scholar]
- Clay K. Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology. 1988;69:10–16. doi:10.2307/1943155 [Google Scholar]
- Craven K.D, Blankenship J.D, Leuchtmann A, Hignight K, Schardl C.L. Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia. 2001;53:44–73. [Google Scholar]
- Eichenseer H, Dahlman D.L. Antibiotic and deterrent qualities of endophyte-infected tall fescue to 2 aphid species (Homoptera, Aphidiae) Environ. Entomol. 1992;21:1046–1051. [Google Scholar]
- Faeth S.H. Are endophytic fungi defensive plant mutualists? Oikos. 2002;99:25–36. doi:10.1034/j.1600-0706.2002.980103.x [Google Scholar]
- Faeth S.H, Bush L.P, Sullivan T.J. Peramine alkaloid variation in Neotyphodium-infected Arizona fescue: effects of endophyte and host genotype and environment. J. Chem. Ecol. 2002;28:1511–1526. doi: 10.1023/a:1019916227153. doi:10.1023/A:1019916227153 [DOI] [PubMed] [Google Scholar]
- Guy P.L. Incidence of Acremonium lolii and lack of correlation with barley yellow dwarf viruses in Tasmanian perennial ryegrass pastures. Plant Pathol. 1992;41:29–34. [Google Scholar]
- Guy P.L, Davis L.T. Variation in the incidence of barley yellow dwarf virus and in the ability of Neotyphodium endophytes to deter feeding by aphids (Rhopalosiphum padi) on Australasian tall fescue. Australas. Plant Pathol. 2002;31:307–308. doi:10.1071/AP02032 [Google Scholar]
- Guy P.L, Johnstone G.R, Morris D.I. Barley yellow dwarf viruses in, and aphids on, grasses (including cereals) in Tasmania. Aust. J. Agric. Res. 1987;38:139–152. doi:10.1071/AR9870139 [Google Scholar]
- Gwinn K.D, Gavin A.M. Relationship between endophyte infestation level of tall fescue seed lots and Rhizoctonia zeae seedling disease. Plant Dis. 1992;76:911–914. [Google Scholar]
- Hamilton C.E, Faeth S.H. Asexual Neotyphodium endophytes in Arizona fescue: a test of the seed germination and pathogen resistance hypothesis. Symbiosis. 2005;38:69–85. [Google Scholar]
- Heie O.E. The Aphidoidea (Hemiptera) of Fennoscandia and Denmark I. Fauna Entomologica Scandinavica. vol. 9. Scandinavian Science Press; Klampenborg, Denmark: 1981. [Google Scholar]
- Hesse U, Latch G.C.M. Influence of Neotyphodium lolii and barley yellow dwarf virus, individually and combined, on the growth of Lolium perenne. Australas. Plant Pathol. 1999;28:240–247. doi:10.1071/AP99039 [Google Scholar]
- Lehtonen P, Helander M, Saikkonen K. Are endophyte-mediated effects on herbivores conditional on soil nutrients? Oecologia. 2005a;142:38–45. doi: 10.1007/s00442-004-1701-5. doi:10.1007/s00442-004-1701-5 [DOI] [PubMed] [Google Scholar]
- Lehtonen P, Helander M, Wink M, Sporer F, Saikkonen K. Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic plant. Ecol. Lett. 2005b;8:1256–1263. doi:10.1111/j.1461-0248.2005.00834.x [Google Scholar]
- Mahmood T, Gergerich R.C, Milus E.A, West C.P, Darcy C.J. Barley yellow dwarf viruses in wheat, endophyte-infected and endophyte-free tall fescue, and other hosts in Arkansas. Plant Dis. 1993;77:225–228. [Google Scholar]
- Rochow W.F, Duffus J.E. Luteoviruses and yellows diseases. In: Kurstak E, editor. Handbook of plant virus infections and comparative diagnosis. Elsevier; Amsterdam, The Netherlands: 1981. pp. 147–170. [Google Scholar]
- Saikkonen K, Faeth S.H, Helander M, Sullivan T.J. Fungal endophytes: a continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998;29:319–343. doi:10.1146/annurev.ecolsys.29.1.319 [Google Scholar]
- Saikkonen K, Ahlholm J, Helander M, Lehtimaki S, Niemelainen O. Endophytic fungi in wild and cultivated grasses in Finland. Ecography. 2000;23:360–366. doi:10.1034/j.1600-0587.2000.d01-1645.x [Google Scholar]
- Saikkonen K, Wäli P, Helander M, Faeth S.H. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004;9:275–280. doi: 10.1016/j.tplants.2004.04.005. doi:10.1016/j.tplants.2004.04.005 [DOI] [PubMed] [Google Scholar]
- SAS Technical Report P-243 1993 SAS/STAT®software: the GENMOD procedure, release 6.09 Cary, NC: SAS Institute Inc.
- Schardl C.L, Phillips T.D. Protective grass endophytes: where are they from and where are they going? Plant Dis. 1997;81:430–438. doi: 10.1094/PDIS.1997.81.5.430. [DOI] [PubMed] [Google Scholar]
- Schardl C.L, Leuchtmann A, Spiering M.J. Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. doi:10.1146/annurev.arplant.55.031903.141735 [DOI] [PubMed] [Google Scholar]
- Siegel M.R. Fungal endophyte-infected grasses—alkaloid accumulation and aphid response. J. Chem. Ecol. 1990;16:3301–3315. doi: 10.1007/BF00982100. doi:10.1007/BF00982100 [DOI] [PubMed] [Google Scholar]
- Siegel M.R, Latch G.C.M. Expression of antifungal activity in agar culture by isolates of grass endophytes. Mycologia. 1991;83:529–537. [Google Scholar]
- Stovall M.E, Clay K. Fungitoxic effects of Balansi cyperi (Clavicipitaceae) Mycologia. 1991;83:288–295. [Google Scholar]
- Wang M.-B, Abbott D.C, Waterhouse P.M. A single copy of a virus-derived transgene encoding hairpin RNA gives immunity to barley yellow dwarf virus. Mol. Plant Pathol. 2000;1:347. doi: 10.1046/j.1364-3703.2000.00038.x. doi:10.1046/j.1364-3703.2000.00038.x [DOI] [PubMed] [Google Scholar]
- Wang R.F, Yang X.W, Ma C.M, Cai S.Q, Li J.N, Shoyama Y.A. Bioactive alkaloid from the flowers of Trollius chinensis. Heterocycles. 2004;63:1443–1448. [Google Scholar]
- Yue Q, Miller C.J, White J.F, Richardson M.D. Isolation and characterization of fungal inhibitors from Epichloë festucae. J. Agric. Food Chem. 2000;48:4687–4692. doi: 10.1021/jf990685q. doi:10.1021/jf990685q [DOI] [PubMed] [Google Scholar]