Abstract
Sexual signals are often critical for mate attraction and reproduction, although their conspicuousness exposes them to parasites and predators. We document the near-disappearance of song, the sexual signal of crickets, and its replacement with a novel silent morph, in a population subject to strong natural selection by a deadly acoustically orienting parasitoid fly. On the Hawaiian Island of Kauai, more than 90% of male field crickets (Teleogryllus oceanicus) shifted in less than 20 generations from a normal-wing morphology to a mutated wing that renders males unable to call (flatwing). Flatwing morphology protects male crickets from the parasitoid, which uses song to find hosts, but poses obstacles for mate attraction, since females also use the males' song to locate mates. Field experiments support the hypothesis that flatwings overcome the difficulty of attracting females without song by acting as ‘satellites’ to the few remaining callers, showing enhanced phonotaxis to the calling song that increases female encounter rate. Thus, variation in behaviour facilitated establishment of an otherwise maladaptive morphological mutation.
Keywords: phonotactic parasitoid, rapid evolution, satellite
1. Introduction
Sexual signals such as colourful plumage are critical for mate attraction and hence reproduction, even though their conspicuousness exposes them to parasites and predators (Zuk & Kolluru 1998). Such signals often represent compromises between natural and sexual selection. Since 1991, we have been examining the responses to such conflicting selective pressure in populations of the field cricket Teleogryllus oceanicus, an Australian and Pacific Island species introduced to three Hawaiian Islands (Oahu, the Big Island of Hawaii and Kauai), where it is subject to an acoustically orienting parasitoid fly, Ormia ochracea (Zuk et al. 1993). The parasitoid is North American in origin and overlaps in range with T. oceanicus only in Hawaii (Lehmann 2003). The fly finds its host using the same signal (the calling song) that males produce to attract mates; fly larvae burrow into the cricket and develop inside, killing the host upon emergence.
Previous work demonstrated that parasitized populations have altered song structure, response to disturbance and calling behaviour compared with unparasitized populations (Zuk et al. 1993, 1995, 1998, 2001; Rotenberry et al. 1996; Lewkiewicz & Zuk 2004). Here we document a much more extreme and rapid adaptive change, near-complete loss of calling, in the Kauai population, and examine its consequences for mate location and the evolution of mate choice in the context of interaction between behavioural plasticity and morphological adaptation.
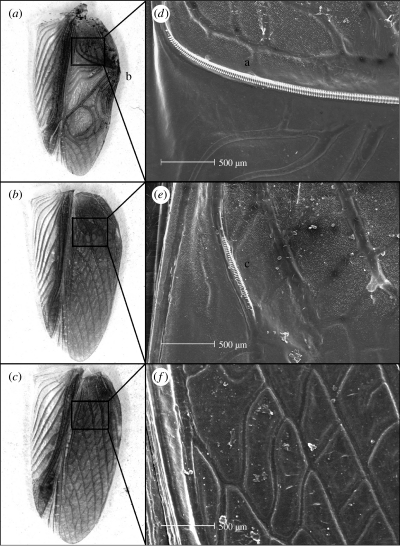
Kauai has always had the highest prevalence of the parasitoid, with nearly 30% of calling males harbouring the fly (Zuk et al. 1993). Presumably owing to the associated mortality, with each field visit since 1991 we heard and observed fewer crickets on that island, and in 2001 only heard a single calling male, with all crickets extremely scarce in intensive searches (methods; see electronic supplementary material). Over a three day visit in 2003, although we heard none calling, crickets were far more abundant than before in their habitat of fields and lawns. Further examination revealed that virtually all Kauai males had female-like wings, lacking the normal stridulatory apparatus of file and scraper required for sound production (hereafter called flatwings; figure 1). Instead, the file is reduced in size and relocated at an angle precluding sound production (figure 1). Flatwings are thus unable to call. Populations from the other Hawaiian Islands as well as descendents from eggs collected on Kauai before 2003 continue to exhibit normal wings.
Figure 1.
Underside of the right forewing from a normal-winged male (a), flatwing male (b) and female (c) Teleogryllus oceanicus. (d–f) SEM micrographs of these wings showing magnified structures of interest. Normal-winged males possess a stridulatory apparatus consisting of the file, a modified Cu2 vein with many evenly spaced (a) teeth and (b) scraper. In flatwing males, a file exists, but is much reduced in size and relocated on the wing precluding (c) sound production. The stridulatory apparatus is absent in females.
Loss of calling clearly protects the crickets from the parasitoid. Although flies are still attracted to sound traps on Kauai, out of 121 flatwings dissected, only one harboured parasitoid larvae, versus greater than 30% infestation rates previously associated with normal-winged males on Kauai. But this protection comes with the price of losing the sexual signal. How do females locate silent flatwing males? Moreover, like most field crickets, T. oceanicus males produce a courtship song after a female is within close range. Females in this and other species require the male to produce the courtship song before mounting to receive a spermatophore (Burk 1983; Libersat et al. 1994). Flatwings can produce neither calling nor courtship song, and crickets are not known to use long-range pheromones for mate location (Tregenza & Wedell 1997). Nevertheless, the now-thriving population of T. oceanicus on Kauai suggests that the obstacles in both detecting and accepting mates have been overcome.
Here, we focus on the difficulty of long-range mate location. We propose that flatwing male T. oceanicus on Kauai behave as ‘satellites’ (Cade 1980) to the few calling males that remain, with an enhanced phonotaxis response to a calling song that brings them into close proximity with the caller. Attracted females should then be much more likely to encounter flatwings as potential mates than they would if the flatwings simply moved at random throughout the habitat. Male crickets from a variety of species, including T. oceanicus, are normally attracted to the song of other males (Kiflawi & Gray 2000), but they usually settle at least 1 m from the caller (M. Zuk 1991–2004, unpublished observations). Satellite behaviour was proposed for a related species, Gryllus texensis, subject to parasitization by O. ochracea in North America (Cade 1975, 1979, 1981). We predicted that flatwings would move towards a caller more quickly, and stop at a point closer to the caller, than would normal-winged males.
2. Material and methods
(a) Collection and survey techniques
We have collected T. oceanicus on Oahu, Kauai and the Big Island of Hawaii regularly since 1991. We localize calling males by sound and thoroughly scan appropriate habitat (lawns and other disturbed areas) for females and non-calling males. Numbers of individuals collected in each sampling effort are shown in electronic supplementary material, table 1. On Kauai, the crickets occur only on lawns around the Kauai Research Station of the University of Hawaii College of Tropical Agriculture.
(b) Field phonotaxis
We performed a field experiment in which 2 m radius circles were delineated within the habitat of crickets on all three Hawaiian Islands. After removing and noting the sex, the wing type and the number of all crickets inside the circle, we played island-specific calling song from a speaker in the centre of the circle. The position, sex and wing morphology of all crickets inside the circle were noted after 20 min, and the distance from all crickets to the speaker was measured.
(c) Song synthesis
We synthesized island-specific calling songs from field-recorded chirps using Canary v. 1.2.4 software. Each song contained the mean values of the following components of T. oceanicus calling song: pulses per long chirp, long chirp pulse duration, long chirp interpulse interval, short chirp pulse duration, short chirp interpulse interval, short chirps per song, pulses per chirp, intersong interval and frequency for songs recorded at 24–26°C (terminology after Otte (1992); see Rotenberry et al. (1996) for sonogram).
3. Results
In 2004, we heard a handful of callers; from a field-collected sample of 133 males, 12 had normal wings, with the remainder being flatwings. Laboratory colonies bred from eggs collected on Kauai since 2003 continue to show flatwing males. No intermediate forms have been observed, and all male crickets on the other two islands with the fly have normal wings (with the exception of four males on Oahu in 2005; see below). This suggests a mutation among male T. oceanicus that invaded the Kauai population in 12–20 generations, assuming four generations per year and a shift between the very late 1990s and 2003. Given the lack of intermediates and the speed at which the population has changed, we suspect that the mutation occurs in one or a very few genes. In an initial study with four replicate matings, we crossed flatwing males with females from a pure-breeding normal-winged population to produce the F1 generation, and crossed F1 siblings to produce a second generation (see electronic supplementary material). Only one replicate produced a substantial number of F2 offspring; the observed ratio of flatwing: normal-winged males in this replicate approximated 1 : 1 (χ2=0.032, p=0.857), as expected if the flatwing trait is controlled by a single sex-linked locus. The observed ratio of flatwing: normal-winged males in the F2 generation was not consistent with an autosomal recessive mode of inheritance (3 : 1; χ2=9.04, p=0.003).
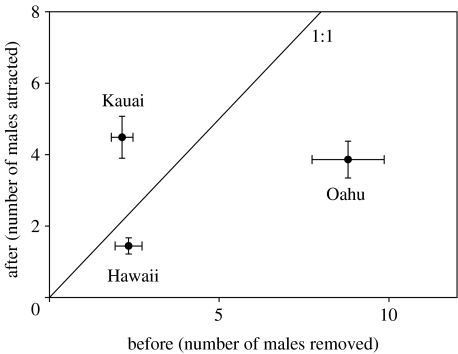
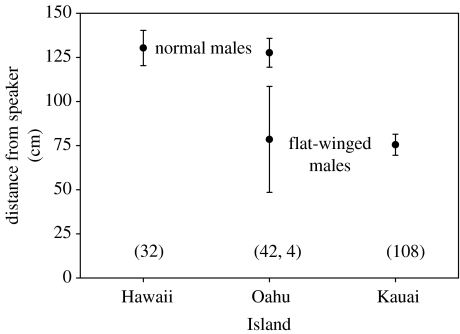
In the field experiment, as predicted, a greater proportion of flatwing males on Kauai was found inside the circle relative to the numbers that had been removed before the trial (figure 2). Kauai males were also significantly closer to the speaker than males on the other two islands (figure 3). Furthermore, four flatwing males were recovered inside the circles on Oahu, and these were found at a mean distance of 78.8 cm from the speaker, virtually identical to the mean distance of 75.5 cm for flatwings on Kauai (figure 3).
Figure 2.
Average (±1 s.e.m.) number of males responding to 20 min playback of island-specific Teleogryllus oceanicus calling songs by moving to within 2 m of a speaker (after) compared to number of males removed from a 2 m radius circle prior to playback (before). After correcting for number of males initially removed, the number attracted differs significantly among islands (ANOVA F2,97=19.35, p<0.001), with Kauai (all flatwings) significantly higher than Oahu (91% normals) and Hawaii (all normal-winged), but the latter not differing between themselves (Tukey's studentized range test).
Figure 3.
Average (±1 s.e.m.) distance that Teleogryllus oceanicus males of two wing morphologies approached speakers playing island-specific calling songs. Sample sizes (number of males) in parentheses. Omitting four flatwings from Oahu owing to small sample size, approach distances vary significantly among islands (ANOVA F2,179=17.95, p<0.001), with Oahu and Hawaii both different from Kauai, but neither different from each other (Tukey's studentized range test). Note that no flatwings occurred in the Hawaii sample and no any normal-winged males in the Kauai sample.
4. Discussion
We document the rapid loss of a sexual signal that we suggest could become established owing to behavioural plasticity. The wing mutation may result from a relatively simple genetic change in a wing development pathway that could well occur in other cricket species and populations, but was able to increase in frequency among Kauai T. oceanicus because behaviour—and strong selection against calling males—allowed it to become advantageous. The behavioural differences between flatwing and normal-winged males may occur because a gene or genes that influenced phonotaxis changed at the same time as the mutation that caused the flatwing morphology. Alternatively, crickets—regardless of wing morphology—may alter their behaviour depending on factors such as population density and success rate of attracting females, so that they are more likely to move towards a caller if they have not attracted a female for some time. Such plasticity has been noted in other cricket species (Hissmann 1990), and may be a more plausible scenario to account for the ability of the crickets to adapt to the rapid change in morphology, because it does not require multiple simultaneous mutations.
How do the flatwings deal with their inability to produce courtship song? Experiments examining the response of females to flatwings versus normal-winged males at close range are underway, but field observations of mating flatwings at speakers suggest that Kauai females at least will accept males without hearing the courtship song, in striking contrast to findings in this and other species where the courtship song is essential to induce mounting by females (Burk 1983; Libersat et al. 1994). Females on islands may have reduced choosiness owing to selection against those females with a narrow range of mate preferences. In small island populations founded by relatively few individuals, choosy females may simply never find an acceptable mate and hence are at a disadvantage compared with less discriminating females (Kaneshiro 1980).
This system is clearly not at equilibrium, and we continue to monitor changes in relative proportions of flatwing and normal-winged males, as well as in a number of parasitoid flies. Although flies are still present on Kauai, it is possible that they will decrease in number and potentially become extinct as host availability declines. We know of no suitable alternative hosts for the fly in Hawaii. Alternatively, if fly populations can persist at low levels, one might expect the generation of frequency-dependent cycles of abundance of flatwing and normal wing types, as natural selection favouring silence causes an increase in flatwings until it is countered by sexual selection favouring calling. Whether this represents a case of West-Eberhard's (2003) version of the idea that ‘behavior takes the lead in evolution’ remains to be seen.
Acknowledgments
We thank A. M. Stoehr, S. N. Gershman and K. Lesser for their comments, Bryoni Yun for assistance with laboratory experiments, the undergraduates who helped in maintaining the crickets, and the Kauai Research Station of the University of Hawaii College of Tropical Agriculture for facilitating fieldwork. We also thank two anonymous reviewers for commenting on the manuscript. This work was supported by the National Science Foundation, the National Geographic Society and the UC Riverside Academic Senate.
Supplementary Material
Crickets were collected on 3 Hawaiian Islands between 1993 and 2005 and examined for wing type. A preliminary breeding experiment examined the mode of inheritance of the flatwing trait.
References
- Burk T. Male aggression and female choice in a field cricket (Teleogryllus oceanicus): the importance of courtship song. In: Gwynne D.T, Morris G.K, editors. Orthopteran mating systems: sexual competition in a diverse group of insects. Westview Press; Boulder, Colorado: 1983. pp. 97–119. [Google Scholar]
- Cade W.H. Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science. 1975;190:1312–1313. [Google Scholar]
- Cade W.H. In: Sexual selection and reproductive competition in insects. Blum M.S, Blum N.A, editors. Academic Press; New York, NY: 1979. pp. 343–379. [Google Scholar]
- Cade W.H. Alternative male reproductive behaviors. Fla. Entomol. 1980;63:30–45. [Google Scholar]
- Cade W.H. Alternative male strategies: genetic differences in crickets. Science. 1981;212:563–564. doi: 10.1126/science.212.4494.563. [DOI] [PubMed] [Google Scholar]
- Hissmann K. Strategies of mate finding in the European field cricket (Gryllus campestris) at different population densities: a field study. Ecol. Entomol. 1990;15:281–291. [Google Scholar]
- Kaneshiro K.Y. Sexual isolation, speciation and the direction of evolution. Evolution. 1980;34:437–444. doi: 10.1111/j.1558-5646.1980.tb04833.x. doi:10.2307/2408213 [DOI] [PubMed] [Google Scholar]
- Kiflawi M, Gray D. Size-dependent response to conspecific mating calls by male crickets. Proc. R. Soc. B. 2000;267:2157–2161. doi: 10.1098/rspb.2000.1263. doi:10.1098/rspb.2000.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann G.U.C. Review of biogeography, host range and evolution of acoustic hunting in Ormiini (Insecta, Diptera, Tachinidae), parasitoids of night-calling bushcrickets and crickets (Insecta, Orthoptera, Ensifera) Zool. Anz. 2003;242:107–120. [Google Scholar]
- Lewkiewicz D.A, Zuk M. Latency to resume calling after disturbance in the field cricket, Teleogryllus oceanicus, corresponds to population-level differences in parasitism risk. Behav. Ecol. Sociobiol. 2004;55:569–573. doi:10.1007/s00265-003-0745-6 [Google Scholar]
- Libersat F, Murray J.A, Hoy R.R. Frequency as a releaser in the courtship song of two crickets, Gryllus bimaculatus (de Geer) and Teleogryllus oceanicus: a neuroethological analysis. J. Comp. Physiol. A. 1994;174:485–494. doi: 10.1007/BF00191714. doi:10.1007/BF00191714 [DOI] [PubMed] [Google Scholar]
- Otte D. Evolution of cricket songs. J. Orthop. Res. 1992;1:25–49. [Google Scholar]
- Rotenberry J.T, Zuk M, Simmons L.W, Hayes C. Phonotactic parasitoids and cricket song structure: an evaluation of alternative hypotheses. Evol. Ecol. 1996;10:233–243. doi:10.1007/BF01237681 [Google Scholar]
- Tregenza T, Wedell N. Definitive evidence for cuticular pheromones in a cricket. Anim. Behav. 1997;54:979–984. doi: 10.1006/anbe.1997.0500. doi:10.1006/anbe.1997.0500 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Zuk M, Kolluru G.R. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 1998;73:415–438. doi:10.1086/420412 [Google Scholar]
- Zuk M, Simmons L.W, Cupp L. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 1993;33:339–343. [Google Scholar]
- Zuk M, Simmons L.W, Rotenberry J.T. Acoustically-orienting parasitoids in calling and silent males of the field cricket Teleogryllus oceanicus. Ecol. Entomol. 1995;20:380–383. [Google Scholar]
- Zuk M, Rotenberry J.T, Simmons L.W. Calling songs of field crickets (Teleogryllus oceanicus) with and without phonotactic parasitoid infection. Evolution. 1998;52:166–171. doi: 10.1111/j.1558-5646.1998.tb05149.x. doi:10.2307/2410931 [DOI] [PubMed] [Google Scholar]
- Zuk M, Rotenberry J.T, Simmons L.W. Geographical variation in calling song of the field cricket Teleogryllus oceanicus: the importance of spatial scale. J. Evol. Biol. 2001;14:731–741. doi:10.1046/j.1420-9101.2001.00329.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crickets were collected on 3 Hawaiian Islands between 1993 and 2005 and examined for wing type. A preliminary breeding experiment examined the mode of inheritance of the flatwing trait.