Abstract
Camouflage is a means to defeat visual detection by predators, whereas visual communication involves a signal that is conspicuous to a receiver (usually a conspecific). However, most intraspecific visual signals are also conspicuous to predators, so that signalling can lead to the serious consequence of predation. Could an animal achieve visual camouflage and simultaneously send a hidden visual message to a conspecific? Here, we present evidence that the polarized aspect of iridescent colour in squid skin is maintained after it passes through the overlying pigmented chromatophores, which produce the highly evolved—and dynamically changeable—camouflaged patterns in cephalopods. Since cephalopods are polarization sensitive, and can regulate polarization via skin iridescence, it is conceivable that they could send polarized signals to conspecifics while staying camouflaged to fish or mammalian predators, most of which are not polarization sensitive.
Keywords: cephalopod, polarization, iridophore, chromatophore, optical signal, skin
1. Introduction
Cephalopods (squid, cuttlefish, octopus) are able to produce a wide variety of body patterns for camouflage and signalling using their optically malleable skin that contains neurally controlled pigmented chromatophores as well as structural light reflectors (Hanlon & Messenger 1996). Squid have two distinct layers in their skin: (i) superficially located chromatophore organs (red, yellow or brown pigments) with radial muscle fibres that are innervated directly by the brain and can thus expand and retract over (ii) underlying iridophore cells. Iridophores are multilayer reflector cells composed of plates of protein interspersed by spaces of cytoplasm (Denton & Land 1971; Crookes et al. 2004, each differing in refractive index. The series of plates and spaces acts as a broadband multilayer interference reflector (Land 1972), one of whose optical properties is the reflectance of polarized light at oblique angles of viewing. Squid can regulate skin iridescence (Hanlon et al. 1990), and therefore the polarized aspect of the reflected light is also changeable. Cephalopods are known to be polarization sensitive (Hanlon & Messenger 1996), an ability that many of their vertebrate predators do not possess (Land & Nilsson 2002). This opens the possibility of a ‘concealed’ communication channel (Shashar et al. 1996) visible only to animals (such as conspecifics) sensitive to polarized light.
2. Materials and results
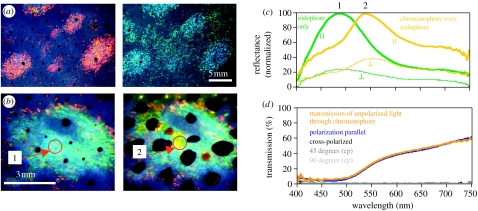
We measured reflectance spectra of skin samples from the mantle of the squid Loligo pealeii using a fibre optic spectrometer (Ocean Optics, USA) attached to a Zeiss dissecting microscope. To analyse polarization, we used linear polaroid filters (Jessops, UK). A diffuse white reflectance standard (Ocean Optics) was used to standardize measurements. The samples were pinned onto a sylgard dish anchored in a goniometer. By positioning the light at 90° to the microscope and tilting the sample by 45° towards the light, we were able to measure reflectance from the skin at 45° incidence (i.e. angle of incident light and angle of reflectance are 45°). Three optical effects were observed. First, there was a distinct colour change from red at normal incidence to blue–green at 45° incidence (figure 1a). Second, the blue–green iridescent reflection (figure 1b no. 1) was linearly polarized (green lines in figure 1c; polarization 72%), as predicted by multilayer theory (Land 1972). Third, pigmented chromatophores acted like filters in the squid's malleable coloration scheme; they altered the colour of light reflected from the underlying iridophores. For example, when a yellow chromatophore expanded over polarized iridophores (figure 1b no. 2), the chromatophore appeared brighter and the spectral peak of the light reflected from the iridophores changed from approximately 480 to 540 nm (figure 1c), yet linearly polarized light was not depolarized when passing upward through the pigmented chromatophore. Polarization decreased only slightly (less than 15%; yellow lines in figure 1c; polarization 61%; n=10 for yellow chromatophores), partly because there is unpolarized light reflected from the pigment granules of the chromatophore organ. Red and brown chromatophores also transmit polarized light when fully expanded; however, when not fully expanded, brown chromatophores block the transmitted light, presumably because the fixed number of pigment granules is more concentrated in the partially expanded pigment organ. Iridophore iridescence and polarization are under physiological control and can be changed within seconds (Hanlon et al. 1990).
Figure 1.
(a) The same iridophore ‘splotches’ viewed at near normal (red reflectance) and 45° incidence (blue–green reflectance) under white light illumination showing the spectral shift when increasing the angle of viewing on multilayer reflectors. (b) Iridophores can be covered within a fraction of a second by overlying, neurally controlled, pigmented chromatophore organs. Figure shows the same iridophore splotch with chromatophores retracted (1) and expanded (2). Arrows and numbers point to the area of spectral measurement shown in (c). (c) Normalized spectral reflectance measurements for both planes of polarization (parallel and perpendicular planes indicated by symbols) of iridophores only (green line peak at ca 480 nm) and yellow chromatophores covering the same iridophores (yellow line peak at ca 540 nm). Chromatophore pigments filter the wavelengths reflected from iridophores, yet linearly polarized light is not depolarized when passing upward through the chromatophore. (d) Transmission of light through yellow chromatophore, showing that chromatophores are neither birefringent nor optically active. Plot shows spectrum of unpolarized light transmitted through chromatophore (orange line), transmission spectrum of chromatophore placed between two polaroid filters in parallel (blue line) as well as when polaroids are crossed (90°; black line). Dark and light grey lines show chromatophore turned by 45° and 90°, respectively, while between crossed polaroids (cp). No transmission of light through cross-polarized, 45° (cp) and 90° (cp).
We also measured polarization through chromatophores, by placing dissected chromatophore layers between two polaroid filters and measuring the light transmitted through the chromatophores. Chromatophores did not alter the degree or the angle of polarization, demonstrating the absence of birefringence and optical activity (figure 1d; n=4).
3. Discussion
Cephalopods are masters of disguise in the animal kingdom. The neurally controlled chromatophore system enables them to quickly camouflage themselves against widely diverse visual backgrounds (Hanlon & Messenger 1996; Messenger 2001). In addition to having a rhabdomeric visual system that allows cephalopods to detect linearly polarized light, they are able to produce polarized skin patterns using iridophores (Shashar & Hanlon 1997), rendering these light-reflecting cells as a possible communication channel (Shashar et al. 1996). Most of the marine vertebrates which prey upon cephalopods do not have polarization vision (Land & Nilsson 2002). Thus, it may be possible for cephalopods to communicate with conspecifics without visual detection by potential predators, while maintaining highly effective camouflage with pigmented chromatophore patterns. In the evolutionary arms race between predator and prey, such a refined scheme would not be totally surprising, and there is some experimental evidence that female cuttlefish receive and react behaviourally to polarized signals shown by conspecifics (Boal et al. 2004).
The notion that animals produce polarization signals and use them in communication, species recognition and mate selection is by no means novel. For example, the wings of butterflies (Helioconius cydno) show polarized patterns that are used for mate recognition (Sweeney et al. 2003). Stomatopod crustaceans are known to have a polarization-sensitive visual system and they produce strongly polarized patterns on parts of their bodies that may be involved in communication (Cronin et al. 2003). Nonetheless, even though polarization signals may be ‘hidden’ from predators that lack polarization sensitivity, most polarization is a side product of iridescence, which in some lighting conditions may be conspicuous to any predator.
To our knowledge, we present the first anatomical evidence for a ‘hidden communication channel’ that can remain masked by typical pigmented camouflage patterns. The polarization signals are not only hidden from animals that are not polarization sensitive, but also squid (cuttlefish and octopus probably as well) could use the overlying pigmented chromatophores to modulate the underlying iridescence, which may be as high as 90% reflectance (L. M. Mäthger & R. T. Hanlon unpublished data), so that they blend into their surroundings with greater refinement of background matching. Behind this camouflage, the polarization signals of the iridescence continue to be sent out, and when viewed against the generally weakly polarized underwater background, these polarization signals will appear conspicuous to polarization-sensitive receivers.
In this paper, we showed that chromatophores are not birefringent and that their pigments (believed to be proteins; Messenger 2001) are probably not arranged in a crystalline form that would affect polarization. Chromatophores were not found to be optically active (NB: optically active means a change in the angle of polarization); however, in an expanded state, chromatophores are very thin (of the order of microns). Such a short optical path length may not likely suffice to reveal optical activity. The mechanism behind the transmission of polarized light by chromatophore pigments warrants further study.
Investigation of this masked polarization signalling system in living squid, cuttlefish and octopus in a variety of natural environments would provide insight into animal camouflage mechanisms and animal visual ecology, and perhaps encourage sensory ecologists to discover similar examples of simultaneous camouflaged signalling.
Acknowledgments
We thank Tom Cronin, Shinya Inoué, Dan-E. Nilsson and two anonymous reviewers for their constructive comments on this manuscript. Special thanks to Phil McFadden whose continued help has greatly improved this work. We are grateful for partial funding from DARPA (DSO) through Anteon contract F33615-03-D-5408.
References
- Boal J.G, Shashar N, Grable M.M, Vaughan K.H, Loew E.R, Hanlon R.T. Behavioral evidence for intraspecific signaling with achromatic and polarized light by cuttlefish (Mollusca: Cephalopoda) Behaviour. 2004;141:837–861. doi:10.1163/1568539042265662 [Google Scholar]
- Cronin T.W, Shashar N, Caldwell R.L, Marshall N.J, Cheroske A.G, Chiou T.-H. Polarization vision and its role in biological signaling. Integr. Comp. Biol. 2003;43:549–558. doi: 10.1093/icb/43.4.549. doi:10.1093/icb/43.4.549 [DOI] [PubMed] [Google Scholar]
- Crookes W.J, Ding L, Huang Q.L, Kimbell J.R, Horwitz J, McFall-Ngai M.J. Reflections: the unusual proteins of squid reflective tissues. Science. 2004;303:235–238. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- Denton E.J, Land M.F. Mechanisms of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. A. 1971;178:43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Hanlon R.T, Messenger J.B. Cambridge University Press; Cambridge, UK: 1996. Cephalopod behaviour. [Google Scholar]
- Hanlon R.T, Cooper K.M, Budelmann B.U, Pappas T.C. Physiological color-change in squid iridophores. I. Behavior, morphology and pharmacology in Lolliguncula brevis. Cell Tissue Res. 1990;259:3–14. doi: 10.1007/BF00571424. doi:10.1007/BF00571424 [DOI] [PubMed] [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. doi:10.1016/0079-6107(72)90004-1 [DOI] [PubMed] [Google Scholar]
- Land M.F, Nilsson D.E. Oxford University Press; Oxford, UK: 2002. Animal eyes. [Google Scholar]
- Messenger J.B. Cephalopod chromatophores:neurobiology and natural history. Biol. Rev. 2001;76:473–528. doi: 10.1017/s1464793101005772. [DOI] [PubMed] [Google Scholar]
- Shashar N, Hanlon R.T. Squids (Loligo pealei and Euprymna scolopes) can exhibit polarized light patterns produced by their skin. Biol. Bull. 1997;193:207–208. doi: 10.1086/BBLv193n2p207. [DOI] [PubMed] [Google Scholar]
- Shashar N, Rutledge P.S, Cronin T.W. Polarization vision in cuttlefish—a concealed communication channel? J. Exp. Biol. 1996;199:2077–2084. doi: 10.1242/jeb.199.9.2077. [DOI] [PubMed] [Google Scholar]
- Sweeney A, Jiggins C, Johnsen S. Polarized light as a butterfly mating signal. Nature. 2003;423:31–32. doi: 10.1038/423031a. doi:10.1038/423031a [DOI] [PubMed] [Google Scholar]