Abstract
Numerous species undergo impressive movements, but due to massive changes in land use, long distance migration in terrestrial vertebrates has become a highly fragile ecological phenomenon. Uncertainty about the locations of past migrations and the importance of current corridors hampers conservation planning. Using archeological data from historic kill sites and modern methods to track migration, we document an invariant, 150 km (one-way) migration corridor used for at least 6000 years by North America's sole extant endemic ungulate. Pronghorn (Antilocapra americana) from the Greater Yellowstone Ecosystem, like other long distant migrants including Serengeti wildebeest (Connochaetes taurinus) and Arctic caribou (Rangifer tarandus), move nearly 50 km d−1, but in contrast to these other species, rely on an invariant corridor averaging only 2 km wide. Because an entire population accesses a national park (Grand Teton) by passage through bottlenecks as narrow as 121 m, any blockage to movement will result in extirpation. Based on animation of real data coupled with the loss of six historic routes, alternative pathways throughout the 60 000 km2 Yellowstone ecosystem are no longer available. Our findings have implications for developing strategies to protect long distance land migrations in Africa, Asia and North America and to prevent the disappearance of ecological phenomena that have operated for millennia.
Keywords: corridors, Pleistocene, pronghorn, Antilocapra americana, migration
1. Introduction
Long distance migration (LDM) in terrestrial vertebrates is an ecological process that has operated globally for thousands, if not millions, of years. Indeed, the possibility of extreme seasonal movements by Alaskan hadrosaurs during the Cretaceous (Hotton 1980) and mammoths during the Late Pleistocene exists although unlikely (Guthrie 1985; Fiorillo & Gangloff 2001). Evidence for Holocene migrations in Bison priscus appears stronger (Guthrie 1990). Nevertheless, in today's crowded world, LDMs are quietly disappearing due to explosive human population growth coupled with massive land use changes. In only 40 years, the long migrations of springbok (Antidorcas marsupialis) and wildebeest (Connochaetes taurinus) in southern Africa have ended (Child & Le Riche 1967; Williamson et al. 1988).
Impediments to movements of wide-ranging terrestrial mammals share common anthropogenic traits: railroad lines for Mongolian gazelles (Procapra gutturosa) in Central Asia (Ito et al. 2005), highways for brown bears (Ursus arctos) in North America (McLellan & Shackleton 1988), agricultural fields for wildebeest in the Serengeti (Serneels & Lambin 2001) and hydroelectric dams for woodland caribou ((Rangifer tarandus) Mahoney & Schaefer 2002). While species like saiga (Saiga tatarica) or chiru (Pantholops hodgsonii) (Schaller 1998; Milner-Gulland et al. 2001) are threatened by poaching, the overarching problem for effective conservation has been large-scale habitat change.
Among the challenges to retain LDM, three biological uncertainties stand out. First, knowledge about how large-bodied species navigate big and remote landscapes remains limited and local pastoralists and critics of habitat protection are often under the notion that migratory mammals simply move elsewhere and find alternative routes. Second, the relationship between specific migration pathways and population viability remains mostly unknown, a problem exacerbated by the historical lack of appropriate technology to identify which lands, if any, are in need of explicit protection. Finally, beyond spatial uncertainty, populations undertaking LDMs exhibit broad inconsistencies over time. When such variation is large and occurs on an annual, decadal or centurial basis, it will be difficult if not impossible to realistically decide which lands are of highest ecological and conservation value.
Here, we report an invariant LDM corridor in North America's sole surviving endemic ungulate, pronghorn (Antilocapra americana). This LDM, at the southern tier of the 60 000 km2 Greater Yellowstone Ecosystem (GYE), winds through geographical bottlenecks that vary in width from 121 to 700 m and has been traversed for at least 6000 years (Miller & Sanders 2000). Irrespective of location, the identification of unusual, historic and inflexible corridors will facilitate conservation efforts on specific lands.
2. Material and methods
To characterize the spatial patterns of migratory pronghorn we analysed a total of 11 450 GPS fixes (based on global positioning system technology; Telonics, Mesa, AZ, USA). Animals were captured with a net from a helicopter in Grand Teton National Park (GTNP), Wyoming. The width of geographical bottlenecks was the estimated maximum distance between fixes for any two individuals during passage. Cross-section dimensions of the migration corridor were obtained first by drawing polygons around all locations within a fixed section and subsequently by averaging the outermost distances at which individuals passed 10 evenly spaced geographical increments along the entire corridor. The XTools Pro extension in ArcMap (DeLaune 2000) was used for estimations.
We then contrasted our results to those of corridor width in migratory wildebeest from the Serengeti by applying the same analytical techniques to data in Thirgood et al. (2004). To do so, we estimated 63 cross-sections of the wildebeest route (range 1.09–74.79 km) using information on all colour-coded individuals. While this approach introduces a source of bias since the coloured points were not stamped by date, our interest was comparative.
3. Results
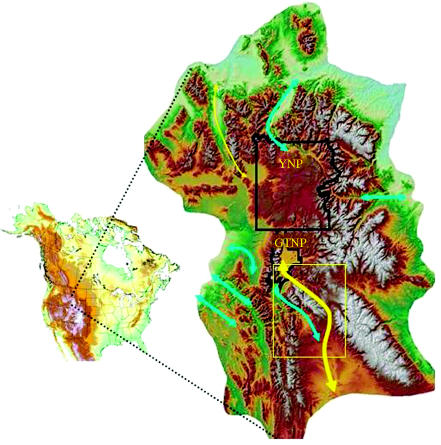
Although pronghorn migration routes in and out of the GYE have been reported (Berger 2004; Sawyer et al. 2005), 6 of 8 routes have been lost (figure 1), due primarily to habitat conversion for agriculture or roads or reservoirs through canyons. Only a single remaining route connects animals that summer in GTNP, a 121 000 ha natural area supporting all native ungulates and carnivores, to suitable wintering areas in the upper Green River basin (figure 1). The narrow corridor appears invariant (figure 2), for all animals that move northward to reach the park used the same pathway though not moving in synchrony and up to one month apart.
Figure 1.
Migration routes (yellow, existing; turquoise, extirpated) of pronghorn in and adjacent to the Greater Yellowstone Ecosystem in relation to yellowstone (YNP) and Grand Teton (GTNP) national parks. Yellow line thickness reflects relative susceptibility to loss. The inset (box) highlights study region of invariant migratory corridor in the upper Green River basin (see figure 2).
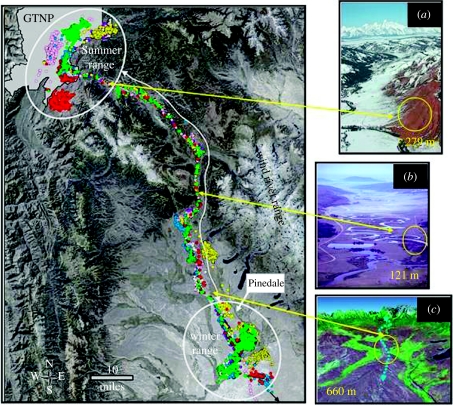
Figure 2.
The migration corridor between GTNP and winter ranges in the upper Green River basin of Wyoming. Dots reflect ca 11 450 points of 10 colour coded adult female pronghorn. Insets (a–c) reflect geographical bottlenecks (yellow circles) and locations along migration route. Enhanced imagery in (c) depicts rivers (green) to east and west of Trapper's Point hunting site where 6000 year old pronghorn bones were recovered.
The longest linear round-trip movement exceeded 560 km and all animals moved through high elevation passes at 2700 m and tapered river valleys. Movements were rapid, involving shifts from summer ranges at ca 2075 m in elevation to slightly higher but less snowy winter ranges at 2370 m approximately 150 km south (figure 2). Autumn migration averaged 3.3 (+2.0 s.e.m.) days. In contrast, spring migration was nine times longer (29.9+6.9 s.e.m. days), as animals followed receding snowlines (see http://www.wcs.org/yellowstone/pronghorn_migration for on-line animation of empirical data on the mechanics of travel through the corridor).
Navigation of the corridor necessitated passage through bottlenecks that varied in width from 100 to 300 m (a–b) to 610 m (c) (figure 2). Historically, the Trapper's Point bottleneck (c) was ca 2000 m wide, tightly constrained by the flow of two rivers and hunted by indigenous Americans during three discrete Mid-Holocene procurement episodes up to about 6000 years ago (Miller & Sanders 2000). Recent residential development has nearly halved the area available for travel through this bottleneck. The mean width used by pronghorn along the entire 150 km route between the GTNP boundary and Trapper's point was 1.91 (+0.118 s.e.m; n=137; range 0.10–5.47) km.
The possibility of adoption of alternate routes is low and neither supported by evidence on the collapse of previously existing pronghorn migrations (figure 1) or our empirical results. For instance, analyses of 16 bi-directional spring and autumn migrations revealed an invariant use of the corridor. Of note is the unsuccessful apparent attempt to use an alternate route (see http://www.wcs.org/yellowstone/pronghorn_migration for on-line animation) during spring migration. After blockage by a highway and multiple efforts to cross a 3500 m mountain chain, a collared female retraced her course and subsequently followed the historic and still functioning corridor to reach summering grounds.
While estimates of corridor width are unavailable for most migratory species, annual variation characterizes chiru and caribou (Schaller 1998; Griffiths et al. 2002). When cross-sections of the route of migratory wildebeest from the Serengeti are contrasted with pronghorn, mean corridor width in the former is substantially greater (34.62; +2.25) km. The ca 18-fold difference in width and the attendant variability during the past few decades (Thirgood et al. 2004), has important implications about how and where to allocate subsequent conservation efforts.
4. Discussion
The availability of GPS technology has enhanced biological knowledge while creating opportunities for conservation, especially for species such as manatees (Trichechus manatus), humpback whales (Megaptera novaeangliae) and African elephants (Loxodonta africana) where conflicts with humans continue to intensify (Wilson et al. 2004; Douglas-Hamilton et al. 2005; Pomilla & Rosenbaum 2005). Although ecological phenomena such as migration are fascinating, challenges to their persistence will arise because of the increasing demand of humans for habitable space. This creates an urgent premium to identify lands crucial for protection.
Our documentation of an invariant migration corridor is noteworthy for two reasons. First, not only is this migration of archeological and cultural importance, but the round-trip movement involves three geographical bottlenecks through which every individual (200–300) of an entire park population must pass. Any obstruction is likely to extirpate pronghorn from GTNP, a supposition bolstered by the loss and failure to re-establish historically used pathways to and from the region's two national parks, Yellowstone and GTNP (inset in figure 1).
Second, the current migration persists in a country with nearly 300 million people and where current national energy policy is reducing biological diversity on public lands (Ehrlich 1994; Berger 2003). For instance, some migrants cross areas that nurture petroleum development, which at full scale will fragment parts of routes that pronghorn have used for millennia. Given the rarity of relict migrations among terrestrial mammals in the Western Hemisphere in excess of even 100 km (Berger 2004) and a desire of most American citizens to maintain a semblance of ecological integrity in national parks (Soule et al. 2003), the invariant route we report that still involves part of an ancient pathway should warrant protective action.
Nevertheless, there are issues of scale, size and ecological function. Unlike the Serengeti with its migratory wildebeest, zebra (Equus burchelli) and Thompson's gazelle (Gazella thomsonii) or Arctic caribou where hundreds of thousands or more move long distances, why should a mere 200–300 migratory pronghorn be of concern? After all, it is common knowledge that there are more pronghorn than people in the State of Wyoming.
Two issues are germane. First, the protection of this migration corridor is more than symbolic. If obstructed, whether by petroleum development, housing or other factors, an entire population from a national park will be eliminated, leaving a conspicuous gap in the function of native predator–prey interactions there. Second, ecological processes are being sacrificed globally, some as a consequence of death by a thousand cuts and others by massive and rapid changes in land use. In an era with few conservation victories, particularly in developed countries, if biological diversity is to be promoted in less-developed countries, we must maintain equal or greater concern for local wildlife conservation by establishing the permanent protection of corridors, whether they are ancient and still functioning or new. Our report of an invariant migratory route suggests that when corridors persist, when they are narrow and when temporal variability in use is low, it should be easier to enact robust conservation measures.
Acknowledgments
We thank the National Park Service, the Rocky Mountain Cooperative Ecological Studies Unit, the Greater Yellowstone Coordinating Committee, K. Tonnessen, M. Maj, B. and N. Weber, R. F. Bowyer, R. Easterbrook, S. and L. Robertson, A. Toivola and the Liz Claiborne and Art Ortenberg Foundation and Wilburforce for support or comments. The GIS Laboratories of the Wildlife Conservation Society, the National Park Service and Sky Truth offered advice and assistance. T. Segerstrom and H. Sawyer have selflessly contributed data and insights. Photos of bottleneck are courtesy of F. Camenzind and J. Catton.
Supplementary Material
This animation depicts the real-time stamped movements of radio-collared animals between summer and winter ranges at three periods: 1) autumn, 2) winter, and spring
References
- Berger J. Is it acceptable to let a species go extinct in a national park? Conserv. Biol. 2003;17:1451–1454. doi:10.1046/j.1523-1739.2003.02467.x [Google Scholar]
- Berger J. The longest mile: how to sustain long distance migration in mammals. Conserv. Biol. 2004;18:320–332. doi:10.1111/j.1523-1739.2004.00548.x [Google Scholar]
- Child G, LeRiche J.D. Recent springbok treks (mass movements) in southwestern Botswana. Mammalia. 1967;33:499–504. [Google Scholar]
- DeLaune, M. G. 2000. XTools Pro ArcMap extension (version 2.0.1. 2003–2004). Redlands: environmental systems research institute.
- Douglas-Hamilton I, Krink T, Vollrath F. Movements and corridors of African elephants in relation to protected areas. Naturwissenschaften. 2005;1 doi: 10.1007/s00114-004-0606-9. (on-line; Springerlink.com) [DOI] [PubMed] [Google Scholar]
- Ehrlich P.H. Energy use and biodiversity loss. Proc. R. Soc. B. 1994;344:99–104. [Google Scholar]
- Fiorillo A.R, Gangloff R.A. The caribou migration model for Arctic hadrosaurs (Dinosauria: Ornithischia): a reassessment. Hist. Biol. 2001;15:323–344. [Google Scholar]
- Griffiths, B., Douglas, D. C., Walsh, N. E., Young, D. D., McCabe, T. R., Russell, D. E., White, R. G., Cameron, R. D. & Whitten, K. R. 2002 The porcupine caribou herd. In Arctic Refuge coastal plain terrestrial wildlife research summaries (ed. D. C. Douglas, P. E. Reynolds & E. B. Rhode). Biological Sciences Report USGS/BRD/BSR-2002-0001, pp. 8–37. US Geological Survey, Biological Resources Division.
- Guthrie R.D. Wooly arguments against the mammoth steppe; a new look at the palynological data. Q. Rev. Archaeol. 1985;6:9–16. [Google Scholar]
- Guthrie R.D. University of Chicago Press; Chicago, IL: 1990. Frozen fauna of the mammoth steppe. [Google Scholar]
- Hotton N. An alternative to dinosaur endothermy. In: Thomas R, Olson E.C, editors. A cold look at warm-blooded dinosaurs. Westview Press; Boulder, CO: 1980. pp. 311–350. [Google Scholar]
- Ito T, Miura N, Lhagvasuren B, Enkhbileg D, Takatsuki S, Tsunekawa A, Jiang Z. Preliminary evidence of a barrier effect of a railroad on the migration of Mongolian gazelles. Conserv. Biol. 2005;19:945–948. doi:10.1111/j.1523-1739.2005.004364.x [Google Scholar]
- Mahoney S.P, Schaefer J.A. Long-term changes in demography and migration of Newfoundland caribou. J. Mammal. 2002;83:957–963. doi:10.1644/1545-1542(2002)083<0957:LTCIDA>2.0.CO;2 [Google Scholar]
- McLellan B.N, Shackleton D.M. Grizzly bears and resource-extraction industries: effects of roads on behavior, habitat use, and demography. J. Appl. Ecol. 1988;25:451–460. [Google Scholar]
- Miller M.E, Sanders P.H. The Trapper's point site (48SU1006): early Archaic adaptations and pronghorn procurement in the upper Green River basin, Wyoming. Plains Anthropol. 2000;45:39–52. doi: 10.1080/2052546.2000.11932022. [DOI] [PubMed] [Google Scholar]
- Milner-Gulland E.J, Kholodova M.V, Bekenov A, Bukreeva O.M, Grachev Yu.A, Amgalan L, Lushchekina A.A. Dramatic decline in saiga populations. Oryx. 2001;35:340–345. doi:10.1046/j.1365-3008.2001.00202.x [Google Scholar]
- Pomilla C, Rosenbaum H.C. Against the current: an inter-ocean whale migration event. Biol. Lett. 2005;1(4):476–479. doi: 10.1098/rsbl.2005.0351. doi:10.1098/rsbl.2005.0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer H.F, Lindzey F, McWhirter D. Mule deer and pronghorn migration in western Wyoming. Wildl. Soc. Bull. 2005;33:1266–1273. [Google Scholar]
- Schaller G.B. University of Chicago Press; Chicago, IL: 1998. Wildlife of the Tibetan steppe. [Google Scholar]
- Serneels S, Lambin E. Impact of land-use change on the wildebeest migration in the northern part of the Serengeti-Mara ecosystem. J. Biogeogr. 2001;28:391–407. doi:10.1046/j.1365-2699.2001.00557.x [Google Scholar]
- Soule M.E, Estes J.A, Berger J, Martinez del Rio C. Ecological effectiveness: conservation goals for interactive species. Conserv. Biol. 2003;17:1238–1250. doi:10.1046/j.1523-1739.2003.01599.x [Google Scholar]
- Thirgood S, et al. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. 2004;7:113–120. doi:10.1017/S1367943004001404 [Google Scholar]
- Williamson D, Williamson J, Ngwamatsoko K.T. Wildebeest migration in the Kalahari. Afr. J. Ecol. 1988;26:269–280. [Google Scholar]
- Wilson B.R, Reid J, Grellier K, Thompson P.M, Hammond P.S. Considering the temporal when managing the spatial: a population range expansion impacts protected areas-based management for bottlenose dolphins. Anim. Conserv. 2004;7:331–338. doi:10.1017/S1367943004001581 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This animation depicts the real-time stamped movements of radio-collared animals between summer and winter ranges at three periods: 1) autumn, 2) winter, and spring