Abstract
Sex ratio evolution relies on genetic variation in either the phenotypic traits that influence sex ratios or sex-determining mechanisms. However, consistent variation among females in offspring sex ratio is rarely investigated. Here, we show that female painted dragons (Ctenophorus pictus) have highly repeatable sex ratios among clutches within years. A consistent effect of female identity could represent stable phenotypic differences among females or genetic variation in sex-determining mechanisms. Sex ratios were not correlated with female size, body condition or coloration. Furthermore, sex ratios were not influenced by incubation temperature. However, the variation among females resulted in female-biased mean population sex ratios at hatching both within and among years.
Keywords: sex ratio, sex allocation, TSD, Ctenophorus pictus
1. Introduction
Sex ratio studies have a long tradition in evolutionary biology. Early explanations for equal sex ratios were later followed by insights into how species- or individual-specific characteristics could lead to biased sex ratios, at both the population level and the individual level (Hardy 2002). Sex ratio evolution is closely linked to the evolution of sex determination, with chromosomal sex determination and random meiosis likely to be a result of selection for equal sex ratios. On the other hand, environmental sex determination frequently leads to biased sex ratios but can be favoured when males and females have different environmental optima (Charnov & Bull 1977).
The fundamental dichotomy of genetic (chromosomal) and environmental sex determinations has recently been challenged (Mittwoch 2005; Sarre et al. 2004). For example, studies of reptiles have documented multiple environmental influences on sex determination in species with heteromorphic chromosomes (Shine et al. 2002). Furthermore, recent progress in avian and mammalian sex ratio studies has shown the ability of females to adjust their sex ratios, suggesting that sex chromosome segregation is not necessarily a random process (West & Sheldon 2002; Sheldon & West 2005). This is often seen as a facultative response to, for example, female condition or paternal quality, but among-family variation in sex ratios could also indicate genetic variation among females in predisposition for sex ratio bias that is more or less independent of the female phenotype or local environmental conditions. However, studies on the repeatability of female effects on offspring sex ratio between breeding attempts are scarce in the sex ratio literature (but see Westerdahl et al. 1997; Oddie & Reim 2002). In the present paper, we report data from a lizard (Ctenophorus pictus) showing strong and consistent effects of female identity on offspring sex ratio.
2. Material and methods
The painted dragon, C. pictus, is a small agamid lizard that occurs at arid and semi-arid habitats in Australia. Females produce two–three clutches per season in natural populations and oviposition occurs from early October to January. Temperature-dependent sex determination has previously been reported in this species (Harlow 2004).
Males and females were captured in Yathong Nature Reserve at the beginning of the mating season in 2004 and 2005. Presence or absence of yellow throat coloration was recorded for each female. Females were housed in pairs in cages (645×413×347 mm), whereas males were kept separately. The cages contained sand, rocks and tiles for basking, a shelter and spinifex grass (Triodia spp.). Under long-term captive conditions, successful reproduction after artificial hibernation has been reported to require continuous presence of the male (Uller & Olsson 2005). However, work in the present laboratory has revealed that this is not required when reproductive animals are brought in from natural populations. Thus, females were introduced into male cages only when receptive (Uller & Olsson 2005). Females were mated to two males before ovulation of each clutch. This procedure could introduce within-clutch variation in offspring traits owing to the variation in paternal genetic composition, but this should not lead to biases in potential effects of female identity. Thus, we consistently used female identity as a random effect in our analyses. The cages were inspected for eggs daily and upon collection, the clutch size, the clutch mass and the female mass were recorded. Mean egg mass was calculated as clutch mass divided by clutch size. Eggs were incubated at constant temperature regimes in plastic boxes filled with moist vermiculite (1 : 7, water : vermiculite volume). The incubation temperatures were set to 26°C in 2004 and to four different temperatures (27.5, 29, 30.5 and 32°C) in 2005. All eggs from each clutch were incubated at the same temperature. The hatchlings were sexed using hemipene eversion. This method reliably confirms sex in most lizards (Harlow 1996), but verification of the accuracy of the method is required for each species. Therefore, we confirmed the sex of 60 offspring upon the emergence of secondary sexual characters (C. pictus is strongly sexually dimorphic), with greater than 97% concordance and, in 2005, sex determination was conducted by two researchers (Tobias Uller & Beth Mott), who reached 100% agreement. Details on the karyotyping methodology can be found in electronic supplementary material.
(a) Statistical analyses
In total, we obtained data from 76 clutches produced by 33 females in 2004 and 76 clutches from 31 females in 2005. Since the studies in 2004 and 2005 were performed on different sets of females and the eggs were incubated at different temperatures, we analysed the years separately. Sex ratios were analysed using repeated measure generalized linear mixed models (GLMM) using the GLIMMIX macro in SAS v. 8.2 with a binomial distribution and a logit link function (Littell et al. 1996). The proportion of males per clutch was denoted as the dependent variable and female identity as the random repeated subject, whereas incubation temperature (in 2005 only), oviposition date and mean egg mass were entered as fixed factors. Three different covariance structures (compound symmetry, unstructured and spatial power) were initially tried for the full models and the models were compared using Akaike information criterion. Compound symmetry proved to result in superior or approximately equal fit as the other covariance structures and was therefore chosen for the analyses of all repeated measures. Random effects and fixed effects were tested using likelihood ratio test and F-test, respectively, and the degrees of freedom were calculated using Satterthwaite's approximation (Littell et al. 1996). Final models were obtained using backward elimination of factors at p>0.25, starting with the highest order interaction. Results are presented as mean±s.e. unless otherwise specified.
3. Results
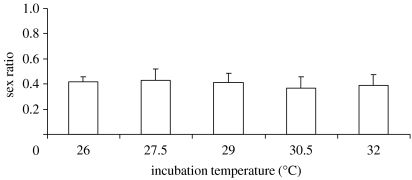
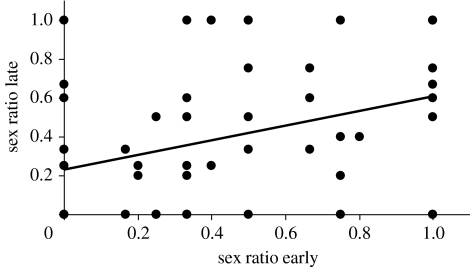
The data from 2005 showed no effect of incubation temperature or oviposition date on offspring sex ratio, but a strong effect of female identity is observed (table 1a; figure 1). This effect was confirmed in the experiment with only one temperature, which also revealed a significant effect of oviposition date (table 1b; increasing sex ratio for later clutches and oviposition date explaining 4% of the variation). The significant variation among females was not explained by any of our phenotypic measures (presence of breeding coloration, SVL and body condition subsequent to oviposition; p>0.25 when included in the model for both the years). The consistent among-family variation in sex ratios over the reproductive season can also be illustrated by plotting the sex ratio of the first clutch produced by a female against the sex ratio of the last clutch produced by the same female, i.e. ignoring intermediate clutches (figure 2, rs=0.37, p=0.006, n=55). The overall population sex ratio during hatching was approximately 0.40 for all treatments and years (figure 1). This bias from 0.50 is statistically significant in both 2004 and 2005 (binomial tests, 2004: 122 males versus 164 females, p=0.015, 95% confidence interval (CI) 0.37–0.49; 2005: 118 males versus 165 females, p=0.005, CI 0.36–0.47). There was no correlation between sex ratios and hatching success (2004: rs=0.09, p=0.42; 2005: rs=0.02, p=0.87). However, if mortality and infertility of eggs were conservatively assigned to make embryos, the deviation from 0.50 was no longer significant (2004: 184 males versus 164 females, p=0.31, CI 0.47–0.58; 2005: 137 males versus 166 females, p=0.11, CI 0.40–0.51).
Table 1.
GLMMs with proportion of males per clutch as the dependent variable for (a) 2005 and (b) 2004. (Values in parentheses denote eliminated factors. All interactions were non-significant (p>0.25) and not shown. See text for model details.)
| (a) 2005 | (b) 2004 | ||||||
|---|---|---|---|---|---|---|---|
| random effects | estimate | χ2 | p | estimate | χ2 | p | |
| female identity | 0.873 | 11.2 | <0.001 | 0.730 | 13.0 | <0.001 | |
| residual | 0.993 | 0.925 | |||||
| fixed effects | n d.f. | d d.f. | F | p | d d.f. | F | p |
|---|---|---|---|---|---|---|---|
| temperature | 3 | 47.5 | (0.47) | (0.70) | — | — | — |
| oviposition date | 1 | 44.4 | (0.01) | (0.93) | 56.2 | 4.04 | 0.049 |
| mean egg mass | 1 | 62.7 | 2.42 | 0.13 | (66.4) | (0.13) | (0.72) |
Figure 1.
Mean sex ratios at five different incubation temperatures (see table 1 for test statistics). Total number of hatchlings per treatment: 26°C, 286; 27.5°C, 67; 29°C, 70; 30.5°C, 66; 32°C, 80.
Figure 2.
Correlation between the sex ratio of the first and that of the last clutch produced by each female over the reproductive season (rs=0.37, p=0.006, n=55).
There was no evidence for heteromorphic sex chromosomes in C. pictus (figure 1 in electronic supplementary material).
4. Discussion
Recent studies on lizards have revealed novel aspects of sex ratio biology (e.g. Shine et al. 2002; Uller et al. 2004; Olsson et al. 2005; Uller & Olsson in press). The present study highlights a number of interesting characteristics with respect to sex ratios in the painted dragon, C. pictus. Harlow's (2004) temperature effects on hatchling sex ratio, based on a relatively small sample, could not be replicated with our extensive design. However, sex ratios were consistently female-biased in both years, in agreement with Harlow (2004), which could not be explained by errors in sex determination. Our data did not suggest any sex-specific mortality, but assigning all infertile eggs and dead embryos to males showed that male-specific mortality could not be excluded as an explanation for the deviation from a 50 : 50 sex ratio. However, deviations from equal sex ratios at the population level can also result from individual sex ratio decisions (e.g. Frank 1987). Indeed, there was a consistent and significant among-female variation in offspring sex ratio among clutches. Importantly, sex ratios were repeatable among clutches within females over the reproductive season, with some females consistently overproducing daughters and some overproducing sons. Potential sources of this variation remain unknown, but it could result from consistent differences among females in some (unknown) phenotypic traits that correlate with sex ratio. For example, maternally transmitted substances (e.g. hormones) to the eggs have been suggested to bias the probability of sexual differentiation towards males or females (Bowden et al. 2000), and such differences among females may remain relatively constant over the reproductive season.
Alternatively, as the sex-determining mechanism is unknown in this species, the among-female variation in sex ratio could be explained by genetic variation in some aspect of sex determination, or a combination of phenotypic and genetic variation in predisposition towards the production of sons versus daughters. For example, the lack of cytogenetically distinct sex chromosomes could suggest a polygenic sex-determining mechanism, as suggested in some fishes (Devlin & Nagahama 2002), which may facilitate retention of genetic variation in sex determination. However, it should be stressed that in other groups of lizards, for example, Lacertidae, where a ZW sex chromosome system has been shown to occur almost universally (Olmo et al. 1987; Odierna et al. 1993), sex chromosomes can evolve rapidly and independently in related taxa (Bosch in den et al. 2003; Odierna et al. 2004). This could be applied in agamids also, where a recent study has found heteromorphic W and Z micro-chromosomes in the bearded dragon, Pogona vitticeps (Ezaz et al. 2005), suggesting that further studies on micro-chromosomes in agamids could reveal novel patterns.
The present variation among females in offspring sex ratio between breeding attempts within years and the recent evidence for multiple modes of sex determination within and among closely related species (Shine et al. 2002; Harlow 2004) suggest exciting future scope for comparative studies of sex ratio evolution in lizards.
Acknowledgments
We are grateful to Ernie Snaith for logistic support and two anonymous reviewers for comments on the paper. Financial support was provided by the Wenner-Gren Foundations (T.U.) and the Australian Research Council (M.O.). All methods adhered to guidelines for the use of animals in research (Animal Ethics permission AE04/03, AE04/04, and AE04/05).
Supplementary Material
Online Appendix 1, including Karyological Methods, Results and Online Fig. 1
References
- Bosch in den H, Odierna G, Aprea G, Barucca M, Canapa A, Capriglione T, Olmo E. Karyological and genetic variation in Middle Eastern lacertid lizards Lacerta laevis and Lacerta kulzeri-complex: a case of chromosomal allopatric speciation. Chromosome Res. 2003;11:165–178. doi: 10.1023/a:1022872016503. doi:10.1023/A:1022872016503 [DOI] [PubMed] [Google Scholar]
- Bowden R.M, Ewert M.A, Nelson C.E. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. B. 2000;267:1745–1749. doi: 10.1098/rspb.2000.1205. doi:10.1098/rspb.2000.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E.L, Bull J.J. When is sex environmentally determined? Nature. 1977;266:828–830. doi: 10.1038/266828a0. doi:10.1038/266828a0 [DOI] [PubMed] [Google Scholar]
- Devlin R.H, Nagahama Y. Sex determination and sex differentiation in fish: and overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. doi:10.1016/S0044-8486(02)00057-1 [Google Scholar]
- Ezaz T, Quinn A.E, Miura I, Sarre S.D, Georges A, Marshall Graves J.A. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res. 2005;13:763–776. doi: 10.1007/s10577-005-1010-9. doi:10.1007/s10577-005-1010-9 [DOI] [PubMed] [Google Scholar]
- Frank S.A. Individual and population sex allocation patterns. Theor. Popul. Biol. 1987;31:47–74. doi: 10.1016/0040-5809(87)90022-0. doi:10.1016/0040-5809(87)90022-0 [DOI] [PubMed] [Google Scholar]
- Hardy I.C.W. Cambridge University Press; Cambridge, UK: 2002. Sex ratios. Concepts and research methods. [Google Scholar]
- Harlow P. A harmless technique for sexing hatchling lizards. Herpetol. Rev. 1996;2:71–72. [Google Scholar]
- Harlow P.S. Temperature-dependent sex determination in lizards. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Institution Press; Washington, DC: 2004. pp. 42–52. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute Inc; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Mittwoch U. Sex is a threshold dichotomy mimicking a single gene effect. Trends Ecol. Evol. 2005;22:96–100. doi: 10.1016/j.tig.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Oddie K.R, Reim C. Egg sex ratio and paternal traits: using within-individual comparisons. Behav. Ecol. 2002;13:503–510. doi:10.1093/beheco/13.4.503 [Google Scholar]
- Odierna G, Kupriyanova L.A, Capriglione T, Olmo E. Further data on sex chromosomes of Lacertidae and a hypothesis on their evolutionary trend. Amphibia–Reptilia. 1993;14:1–11. [Google Scholar]
- Odierna G, Aprea G, Capriglione T, Puky M. Chromosomal evidence for the double origin of viviparity in the European common lizard, Lacerta (Zootoca) vivipara. Herpetol. J. 2004;14:157–160. [Google Scholar]
- Olmo E, Odierna G, Capriglione T. Evolution of sex-chromosomes in lacertid lizards. Chromosoma. 1987;96:33–38. doi:10.1007/BF00285880 [Google Scholar]
- Olsson M, Madsen T, Uller T, Wapstra E, Ujvari B. The role of Haldane's rule in sex allocation. Evolution. 2005;59:221–225. doi:10.1554/04-474 [PubMed] [Google Scholar]
- Sarre S.D, Georges A, Quinn A. The ends of a continuum: genetic and temperature-dependent sex determination in reptiles. BioEssays. 2004;26:639–645. doi: 10.1002/bies.20050. doi:10.1002/bies.20050 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2005;163:40–54. doi: 10.1086/381003. doi:10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Shine R, Elphick M, Donellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 2002;5:486–489. doi:10.1046/j.1461-0248.2002.00351.x [Google Scholar]
- Uller T, Olsson M. Continuous male presence required for fertilisation in captive painted dragons, Ctenophorus pictus. J. Exp. Zool. 2005;303A:1115–1119. doi: 10.1002/jez.a.232. doi:10.1002/jez.a.232 [DOI] [PubMed] [Google Scholar]
- Uller, T. & Olsson, M. In press. No seasonal sex ratio shift despite sex-specific fitness returns of hatching date in a lizard with GSD. Evolution [PubMed]
- Uller T, Massot M, Richard M, Lecomte J, Clobert J. Long-lasting fitness consequences of prenatal sex ratio in a viviparous lizard. Evolution. 2004;58:2511–2516. doi: 10.1111/j.0014-3820.2004.tb00880.x. doi:10.1554/04-355 [DOI] [PubMed] [Google Scholar]
- West A.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. doi:10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T. Sex ratio variation among broods of great reed warblers. Mol. Ecol. 1997;6:543–548. doi:10.1046/j.1365-294X.1997.00217.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Appendix 1, including Karyological Methods, Results and Online Fig. 1