Abstract
Before the appearance of a functional heart in many invertebrate species, the assumption was that general body movements provide circulatory function. Consequently, I investigated the frequency of gut movements in the brine shrimp, Artemia franciscana, immediately post-hatch to the point when a functional heart appeared. Prior to cardiac ontogeny, movements of internal musculature and gut provided pre-cardiac circulatory currents with the rate of gut movements increasing when swimming limbs were impeded. There was also some evidence that gut movements were responsive to low oxygen, indicating a possible regulatory function for the gut in early circulation. Overall, this suggests that general body movements are not always adequate to provide internal circulation in small, heartless individuals.
Keywords: invertebrate circulation, cardiac ontogeny, extracardiac circulation, comparative developmental physiology
1. Introduction
Central to circulatory function, in many animal groups, is the presence of a specialized pump, the heart (Harvey 1628; McMahon et al. 1997a,b). This said, in a number of (often small) invertebrates which lack a heart (e.g. flatworms, nematodes, some annelids, some copepods, barnacles, rotifers), and in the early developmental stages of species where a heart is present in the adult, but not yet developed (e.g. some fishes, some crustaceans), it is widely believed that general body movements are sufficient to ensure mixing of blood/haemolymph (e.g. Lereboullett 1850; Maynard 1961; Burggren & Pinder 1991; McMahon et al. 1997a,b). Unfortunately, such an intuitive idea is not the object of experimental verification.
The development of circulatory function in invertebrates has attracted considerable attention recently, with an emphasis on the control mechanisms involved as well as on when and how cardiac function commences (McMahon et al. 1997a,b; Reiber & Harper 2001; Spicer 2001; Harper & Reiber 2004). While cardiac formation in arthropod crustaceans is nearly always intimately associated with the ontogeny of thoracic segmentation, in many precocial species, a functioning heart does not appear until a considerable time after hatching, e.g. the brine shrimp Artemia franciscana, the shrimp Metapeneaus ensis and the krill Meganyctiphanes norvegica (Spicer 1994, 2001; McMahon et al. 2002). The assumption has been that prior to cardiac ontogeny, and in common with other small invertebrates, general body movements (in the case of Artemia, particularly the movement of the complex array of somatic muscles attached to the second maxillipeds, which are primarily responsible for locomotion in these early stages) provide circulatory function, with some suggestions that intestinal movements might also be important in regulating pre-cardiac circulatory function (Maynard 1961; Spicer 1994).
Consequently, I investigated the frequency of gut movements (fGs), post-hatch to the point when a functional heart appeared, in individual brine shrimp A. franciscana, in which the swimming limbs either beat unimpeded or had been prevented from moving. The prediction was that, if the movements of somatic muscles (mainly associated with limb beating) and gut were important in regulating circulation before the appearance of the heart, then physically impeding swimming movements would be accompanied by a compensatory increase in the gut movement(s). In a second experiment, I examined the effect of low-environmental oxygen tension (PO2) on individual's gut movements, prior to cardiac formation. Here, I predicted that either or both limb beating and gut movements would increase in frequency to compensate for the internal PO2 decrease, linked to a decline in the environmental PO2. I present here the first experimental evidence for the role of the gut as an extracardiac circulatory organ open to environmental modulation, and acting before the heart appears in a developing invertebrate.
2. Material and methods
To provide all the different developmental stages for the experiments described later, brine shrimp were cultured exactly as described in Spicer (1994). Developmental stage for early stages was assigned following Anderson (1967; stages A1–A4). However, Anderson's scheme does not cover all the developmental stages examined here. Therefore, once individuals had began to build a thorax (A4), the scheme of Weisz (1947; W), based on the number of segments present, was followed. Thus, W6 represents a stage, where there are six completely formed segments and so on (right-hand image of figure 1).
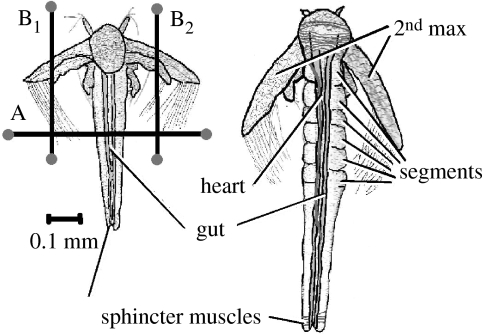
Figure 1.
Diagram of two developmental stages of Artemia (Anderson stage 2 (A2) on the left and Weiss stage 6 (W6) on the right) showing key features referred to in the text, how individuals were restrained within the field of view (A) and how their second maxillipeds were impeded (B1 and B2).
To investigate the importance of both the muscles responsible for producing swimming movements (second antennae) and the gut movements in creating an internal circulation, and how this changed with the onset of cardiac activity (measure as heart rate, fH), the following experiment was carried out. Individuals were carefully transferred to a water droplet (approx. 0.3 ml) contained in an indented, transparent microscope stage thermostated at 25 °C. Cardiac and gut activities, together with indications of circulatory movement within the body (e.g. movements of haemolymph cells), were recorded using a video camera (JVC1/2 inch CCD model no. TK-C1381) coupled to a variable playback speed video recorder (BR-S925E time lapse VHS, JVC) mounted on a microscope (SDZ-PL, Kyowa). Examination of the material took place under low-power magnification (×10–40) using a cold light source.
Individuals were restrained within the field of view by stretching a fine human hair (infant, blonde) tightly across the thorax (marked ‘A’ in the left-hand image of figure 1) and left for 40 min before recordings were made. The hair was held in place using small amounts of Blu Tack. This successfully restrained the individual without impeding the locomotor movements of the second maxillipeds. This recording and restraining technique was previously used to investigate the cardiac activity in Artemia, and was shown to yield similar results with more sophisticated techniques employing micro flow-through systems (Spicer 1994). In order to impede the beating of the second maxillipeds, two more hairs were used, one for each maxilliped (marked ‘B1’ and ‘B2’ in the left-hand side of figure 1). Again, individuals were left for 40 min under these conditions before any recordings were made.
To investigate the effect of reduced PO2 and restriction of limb movement on the fG, the experiment described earlier was repeated for individuals at the developmental stage A3 (i.e. prior to cardiac ontogeny) with the following modifications. Conditions of reduced PO2 (6–7 kPa) were imposed by continuously passing a humidified gas mixture, constructed using a set of precision gas-mixing pumps (Wostoff, Bochum, Germany), gently over the water contained on the microscope stage. Individuals were exposed to reduced PO2 for 1 h before any recordings were made. The PO2 of the water was verified in early experiments (using a polarographic O2 electrode (model 1302, Strathkelvin Instruments, Glasgow) coupled to a computer-controlled O2 meter (model 928, Strathkelvin Instruments, Glasgow)), before and after measurements of gut and cardiac activity were recorded. Equilibration occurred within 5 min and the water PO2 remained constant thereafter. Control experiments were carried out in normoxia (PO2=20 kPa), produced by the gas-mixing apparatus. Restriction of limb movement was carried out exactly as described for the first experiment. Additionally, the frequency of limb beating under normoxic and hypoxic conditions (in individuals with unrestricted limbs) was quantified.
3. Results
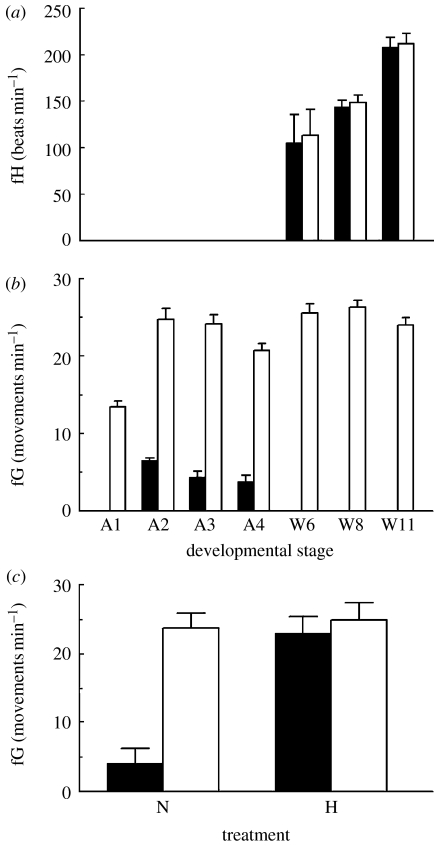
The onset of cardiac function was observed in W6 individuals. Initially, individual fH was irregular, but quickly became regular within a few hours. Cardiac activity increased significantly with development (F2,59=34.5, p<0.001), but was unaffected by impeding the swimming limbs (F1,59=0.06, p>0.05; figure 2a). Gut movements commenced in stage A2 individuals (figure 2b). These ‘whip-like’ movements that travel along the length of the lower gut were quite distinct from the small peristaltic waves that travel continually along the gut, arising at the anus and travelling in an anterior direction. Initial experiments indicated that these smaller peristaltic waves were not affected by either limb impediment or exposure to reduced PO2. Once present, the fG decreased with the increasing development (ANOVA F2,29=6.61, p=0.005). The cessation of gut movements in stage W6 individuals coincided with the appearance of a functional heart (figure 2a,b) and also with the breakdown of the partition between the midgut and hindgut, such that the gut was open and feeding could commence.
Figure 2.
Changes in (a) heart rate (fH) with development, (b) gut movements (fG) with development and (c) gut movements in Anderson stage 3 individuals under normoxic (N) and hypoxic (H) conditions. Individuals with unimpeded swimming limbs (filled bars); individuals with impeded swimming limbs (clear bars). All values are mean+s.d. with n=10 except in the case of (c), where n=20.
Gut movements were present in individuals of all the developmental stages examined when their second maxillipeds were impeded (figure 2b). For all the developmental stages, fG in individuals with limbs impeded greatly exceeded that with unimpeded limbs, such that no formal test of difference was required (or indeed was appropriate, given that no gut movements were recorded for half of the stages examined). There was a significant effect of development on fG in limb-impeded individuals (ANOVA F6,69=13.64, p<0.001), largely attributable to an increase in the rate between the developmental stages A1 and A2. Thereafter, fG in limb-impeded individuals did not vary significantly and remained in the range of 20.7–26.1 movements per minute.
In the second experiment, the pattern of gut and cardiac activities for hypoxic and normoxic individuals (developmental stage A3), with either restrained or unrestrained swimming limbs, is shown in figure 2c. Two-way ANOVA showed that there was a significant effect of restraining the swimming limbs (F1,75=406.7, p<0.001). There was also an effect of environmental hypoxia (F1,75=330.9, p<0.001) and a significant interaction term (F1,75=291.7; p<0.001). Reduced PO2 produced the same elevated fGs as restricting the limbs of these shrimps did. In each case, detailed visual examination of the haemocoel showed haemolymph movements that corresponded with the gut movements. Student's t-test showed that there was no significant effect of reduced PO2 on the beating of the swimming limbs of shrimps (p>0.05), which remained constant at 743.6±34.2 beats per minute.
4. Discussion
Before the advent of a functional heart in Artemia, circulatory function is provided by both general body movements (mainly via the somatic muscles attached to second maxillipeds) and environmentally modulated, large-scale gut movements. Internal hypoxia (i.e. reduced PO2), generated as a result of impeding swimming movements or imposed environmental hypoxia, resulted in an increase in the fGs in each of the early developmental stages investigated here. The cessation of these large-scale gut movements coincided with the ontogeny of cardiac activity although such movements could still be induced by internal hypoxia. While gut peristalsis has long been recognized as a feature of the gut in embryonic, larval and adult crustaceans (MunroFox 1952, Maynard 1961, Berrill 1971), the ‘whip-like’ movement recorded here has not. That there are two different types of gut movement may explain past contradictory interpretations of gut activity (see Maynard 1961 for review).
All these together constitute the first experimental evidence for a regulatory function in the use of the gut as an extracardiac engine of circulation in an invertebrate, prior to cardiac ontogeny. This suggests that, in contrast to the popular belief, general body movements are not always adequate to provide internal circulation in small, heartless individuals.
References
- Anderson D.T. Larval development and segment formation in the branchiopod crustaceans Limnadia stanleyana and Artemia salina. Aust. J. Zool. 1967;15:47–91. doi:10.1071/ZO9670047 [Google Scholar]
- Berrill M. The embryonic behaviour of Caprella unica (Crustacea: Amphipoda) Can. J. Zool. 1971;49:499–504. doi: 10.1139/z71-076. [DOI] [PubMed] [Google Scholar]
- Burggren W.W, Pinder A.W. Ontogeny of cardiovascular and respiratory physiology in lower vertebrates. Annu. Rev. Physiol. 1991;53:107–135. doi: 10.1146/annurev.ph.53.030191.000543. doi:10.1146/annurev.ph.53.030191.000543 [DOI] [PubMed] [Google Scholar]
- Harper S.L, Reiber C.L. Physiological development of the embryonic and larval crayfish heart. Biol. Bull. 2004;206:78–86. doi: 10.2307/1543538. [DOI] [PubMed] [Google Scholar]
- Harvey W. Blackwell; Oxford, UK: 1628. Exercitatio Anatomica de Motu Cordis et Sanguinis in Animalibu. [Google Scholar]
- Lereboullett A. Observations anatomiques et physiologiques. Mém. Soc. Mus. Hist. Nat. Strasbourg. 1850;4:211–253. [Google Scholar]
- Maynard D.M. Circulation. In: Waterman T.H, editor. The physiology of Crustacea. vol. 1. Academic Press; New York, NY: 1961. pp. 161–214. [Google Scholar]
- McMahon B.R, Bourne G.B, Chu K.H. Invertebrate cardiovascular development. In: Burggren W.W, Kelly B.B, editors. Cardiovascular development: molecules to organisms. Cambridge University Press; Cambridge, UK: 1997a. pp. 127–144. [Google Scholar]
- McMahon B.R, Wilkens J.L, Smith P.J.S. Invertebrate circulatory systems. In: Danzler W.H, editor. Handbook of physiology. Comparative physiology. vol. II. American Physiological Society; Bethesda, MD: 1997b. pp. 931–1008. Section 13. [Google Scholar]
- McMahon B.R, Tanaka K, Doyle J.E, Chu K.H. A change of heart: cardiovascular development in the shrimp Metapenaeus ensis. Comp. Biochem. Physiol. 2002;133:577–587. doi: 10.1016/s1095-6433(02)00196-4. doi:10.1016/S1095-6433(02)00196-4 [DOI] [PubMed] [Google Scholar]
- MunroFox H. Anal and oral intake of water by Crustacea. J. Exp. Biol. 1952;29:583–599. [Google Scholar]
- Reiber C.L, Harper S.L. Perspectives on cardiac physiological ontogeny in crustaceans. Zoology. 2001;104:103–113. doi: 10.1078/0944-2006-00013. doi:10.1078/0944-2006-00013 [DOI] [PubMed] [Google Scholar]
- Spicer J.I. Ontogeny of cardiac function in the brine shrimp Artemia franciscana Kellogg 1906 (Branchiopoda: Anostraca) J. Exp. Zool. 1994;270:508–516. doi: 10.1002/jez.1402700604. doi:10.1002/jez.1402700604 [DOI] [PubMed] [Google Scholar]
- Spicer J.I. Development of cardiac function in crustaceans: pattern, processes. Am. Zool. 2001;41:1068–1077. [Google Scholar]
- Weisz P.B. The histological pattern of metameric development in Artemia salina. J. Morphol. 1947;81:45–89. doi: 10.1002/jmor.1050810103. doi:10.1002/jmor.1050810103 [DOI] [PubMed] [Google Scholar]