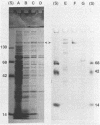
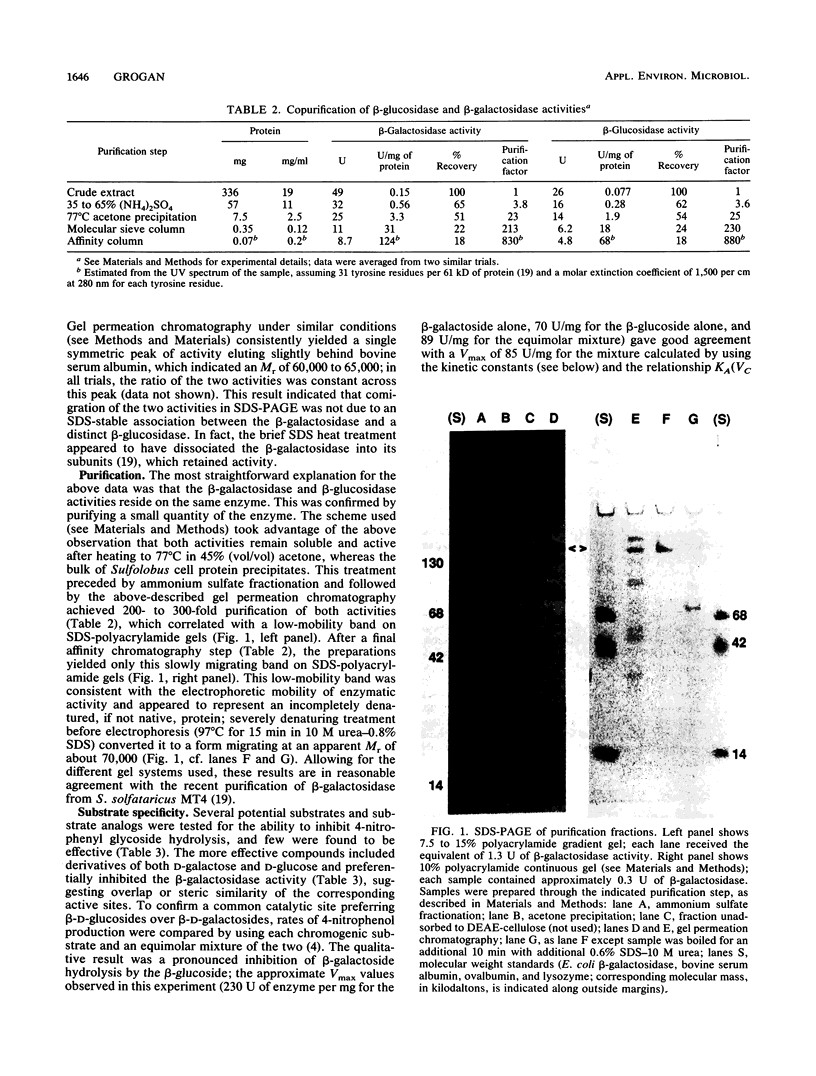
Abstract
A survey of Sulfolobus isolates showed all to contain thermostable enzyme activities hydrolyzing various glycosidic compounds. Of those not previously reported, the β-glucosidase activity of Sulfolobus solfataricus isolate P2 was chosen for further study and found to have the same kinetics of inactivation, apparent molecular weight, and many (though not all) other biochemical properties of the β-galactosidase also present in this strain. The two activities copurified approximately 850-fold to apparent homogeneity. The enzyme, whose subunit Mr was estimated to be 60,000 to 65,000 by gel permeation chromatography of the active enzyme and 70,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the denatured form, hydrolyzed a variety of low-molecular-weight, β-linked glycosides and could account for most of the corresponding activities found in crude extract. Kinetic analyses indicated that chromogenic β-d-galactosides and β-d-glucosides are hydrolyzed at a common active site and that β-glucosides and β-fucosides represent the preferred substrates. The liberation of aglycone from aryl β-d-glucosides was stimulated by alcohols in a manner suggesting specific interaction between alcohol and enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigad G. Colorimetric ultramicro assay for reducing sugars-1. Methods Enzymol. 1975;41:27–29. doi: 10.1016/s0076-6879(75)41006-0. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Chinchetru M. A., Cabezas J. A., Calvo P. Purification and characterization of a broad specificity beta-glucosidase from sheep liver. Int J Biochem. 1989;21(5):469–476. doi: 10.1016/0020-711x(89)90126-2. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Rozzo C., Montecucchi P., Rossi M. Isolation and sequencing of a new beta-galactosidase-encoding archaebacterial gene. Gene. 1990 Sep 28;94(1):89–94. doi: 10.1016/0378-1119(90)90472-4. [DOI] [PubMed] [Google Scholar]
- Goodman R. E., Pederson D. M. beta-Galactosidase from Bacillus stearothermophilus. Can J Microbiol. 1976 Jun;22(6):817–825. doi: 10.1139/m76-118. [DOI] [PubMed] [Google Scholar]
- Grogan D. W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989 Dec;171(12):6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan D., Palm P., Zillig W. Isolate B12, which harbours a virus-like element, represents a new species of the archaebacterial genus Sulfolobus, Sulfolobus shibatae, sp. nov. Arch Microbiol. 1990;154(6):594–599. doi: 10.1007/BF00248842. [DOI] [PubMed] [Google Scholar]
- Guagliardi A., Manco G., Rossi M., Bartolucci S. Stability and activity of a thermostable malic enzyme in denaturants and water-miscible organic solvents. Eur J Biochem. 1989 Jul 15;183(1):25–30. doi: 10.1111/j.1432-1033.1989.tb14891.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Gambacorta A., DeRosa M., Gulik A. Polar lipids of thermophilic prokaryotic organisms: chemical and physical structure. Annu Rev Biophys Biophys Chem. 1987;16:25–47. doi: 10.1146/annurev.bb.16.060187.000325. [DOI] [PubMed] [Google Scholar]
- Pisani F. M., Rella R., Raia C. A., Rozzo C., Nucci R., Gambacorta A., De Rosa M., Rossi M. Thermostable beta-galactosidase from the archaebacterium Sulfolobus solfataricus. Purification and properties. Eur J Biochem. 1990 Jan 26;187(2):321–328. doi: 10.1111/j.1432-1033.1990.tb15308.x. [DOI] [PubMed] [Google Scholar]
- Plant A. R., Oliver J. E., Patchett M. L., Daniel R. M., Morgan H. W. Stability and substrate specificity of a beta-glucosidase from the thermophilic bacterium Tp8 cloned into Escherichia coli. Arch Biochem Biophys. 1988 Apr;262(1):181–188. doi: 10.1016/0003-9861(88)90180-4. [DOI] [PubMed] [Google Scholar]
- Pulvin S., Friboulet A., Thomas D. Substrate inhibition or activation kinetics of the beta-galactosidase from the extreme thermoacidophile archaebacterium Caldariella acidophila. Biochim Biophys Acta. 1990 Nov 15;1041(2):97–100. doi: 10.1016/0167-4838(90)90050-p. [DOI] [PubMed] [Google Scholar]
- Steers E., Jr, cuatrecasas P. Isolation of beta-galactosidase by chromatography. Methods Enzymol. 1974;34:350–358. doi: 10.1016/s0076-6879(74)34037-2. [DOI] [PubMed] [Google Scholar]
- Ulrich J. T., McFeters G. A., Temple K. L. Induction and characterization of -galactosidase in an extreme thermophile. J Bacteriol. 1972 May;110(2):691–698. doi: 10.1128/jb.110.2.691-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]