Abstract
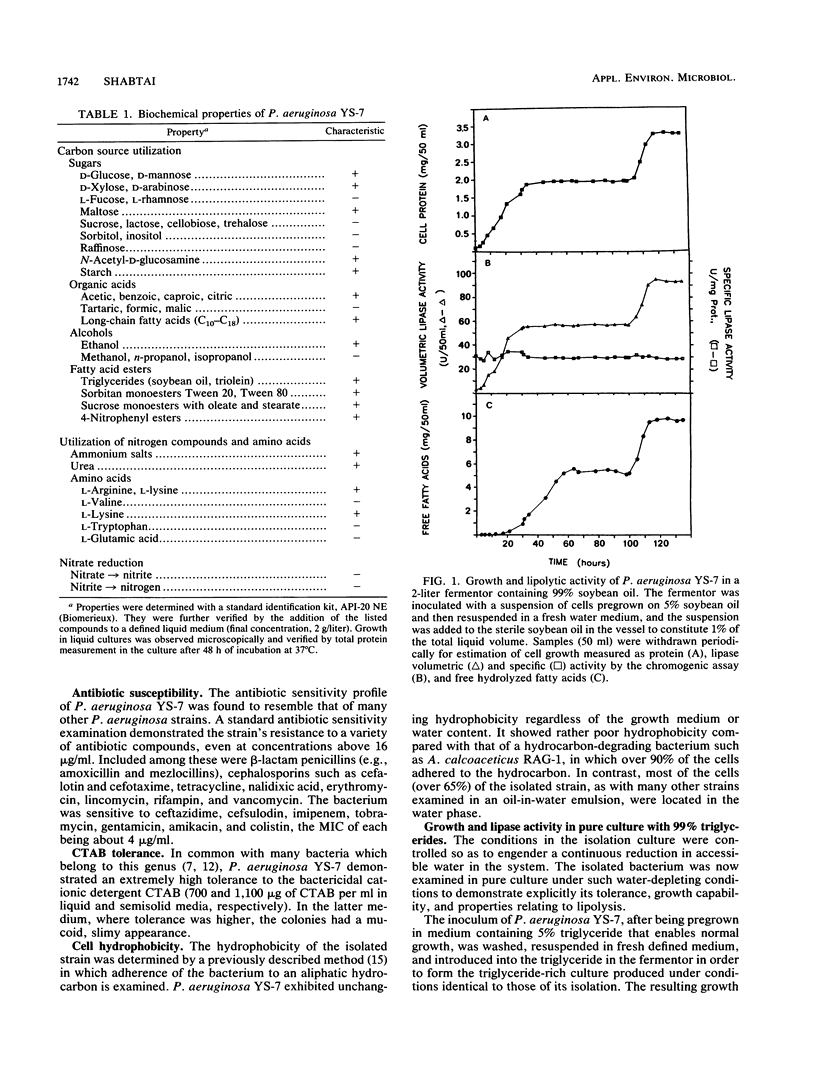
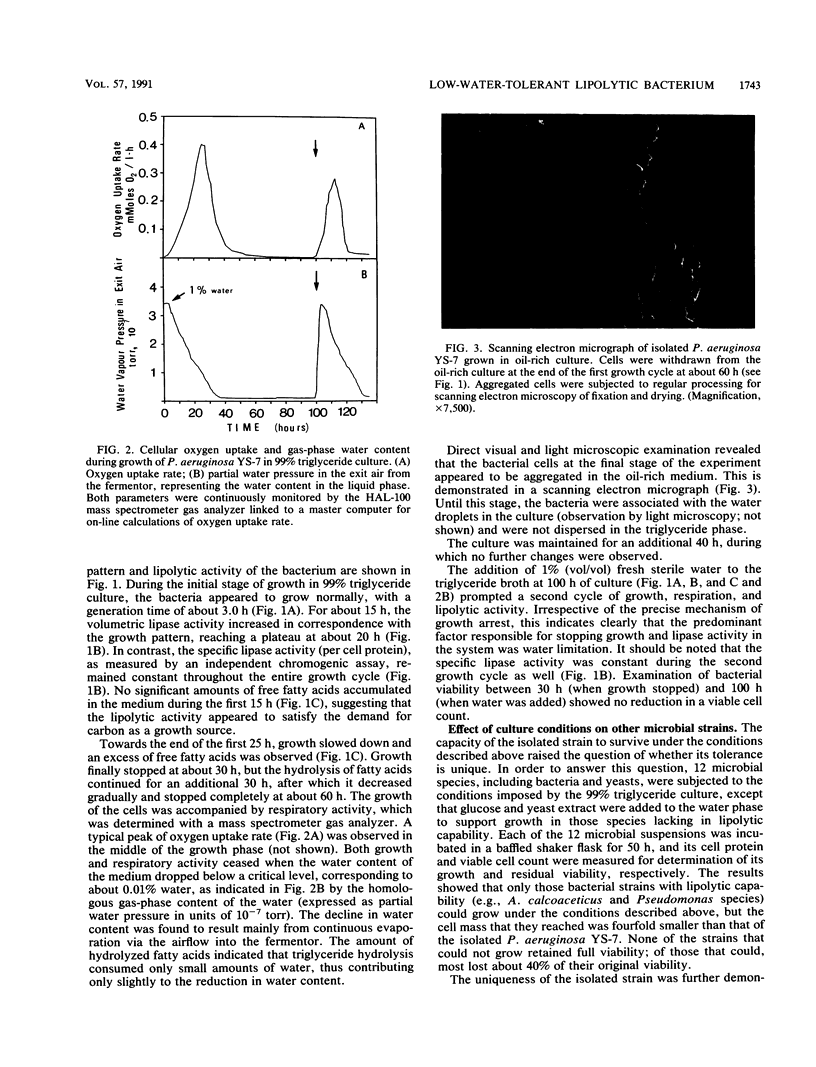
A unique lipolytic bacterium was isolated in a selective growth system consisting of 99% triglycerides and a 1% water phase. The bacterium, termed Pseudomonas aeruginosa YS-7, was able to grow in an environment of low water content and could also survive amphipathic, osmotic, and matrical water stress in a triglyceride-rich culture. The isolated strain was identified as P. aeruginosa on the basis of standard physiological, biochemical, and serological assays. The strain is a gram-negative motile rod, aerobic, pigment forming, and capable of growing at 42 degrees C. It is highly tolerant of high concentrations of the cationic detergent cetyltrimethylammonium bromide and of the fatty acid salts derived from bacterial hydrolysis of the oil. Growth of the bacterium in a pure culture in a 99% triglyceride medium lasted until most of the water was evaporated or consumed. Growth was accompanied by triglyceride hydrolysis, which continued to occur even after growth saturation until the water was totally depleted. No loss of viability was observed when the culture was maintained under water-depleted conditions for an additional 40 h. A second cycle of bacterial growth and triglyceride hydrolysis was immediately initiated upon the addition of 1% (vol/vol) water to the culture. Lipase activity was stable regardless of changes in culture conditions. The isolated strain is uniquely resistant to severe water stress in a triglyceride-rich medium or under cold acetone precipitation compared with 12 other microbial strains, including bacteria and yeasts. Among these 12, only the lipolytic strains grew in the 99% triglyceride medium, but they reached a cell mass fourfold smaller than that of P. aeruginosa YS-7.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briozzo J., de Lagarde E. A., Chirife J., Parada J. L. Effect of water activity and pH on growth and toxin production by Clostridium botulinum type G. Appl Environ Microbiol. 1986 Apr;51(4):844–848. doi: 10.1128/aem.51.4.844-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcott P. H., Calcott K. N. Involvement of outer membrane proteins in freeze--thaw resistance of Escherichia coli. Can J Microbiol. 1984 Mar;30(3):339–344. doi: 10.1139/m84-050. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. On the survival of frozen bacteria. J Gen Microbiol. 1961 Nov;26:367–378. doi: 10.1099/00221287-26-3-367. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Salonius P. O., Chase F. E. A note on the differential response of arthrobacter spp. and pseudomonas spp. to drying in soil. Can J Microbiol. 1965 Aug;11(4):746–748. doi: 10.1139/m65-100. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., McCoy C. J. Epoxidation of 1,7-octadiene by Pseudomonas oleovorans: fermentation in the presence of cyclohexane. Appl Environ Microbiol. 1977 Jul;34(1):47–49. doi: 10.1128/aem.34.1.47-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabtai Y., Gutnick D. L. Enhanced emulsan production in mutants of Acinetobacter calcoaceticus RAG-1 selected for resistance to cetyltrimethylammonium bromide. Appl Environ Microbiol. 1986 Jul;52(1):146–151. doi: 10.1128/aem.52.1.146-151.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabtai Y., Gutnick D. L. Tolerance of Acinetobacter calcoaceticus RAG-1 to the cationic surfactant cetyltrimethylammonium bromide: role of the bioemulsifier emulsan. Appl Environ Microbiol. 1985 Jan;49(1):192–197. doi: 10.1128/aem.49.1.192-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabtai Y. Production of exopolysaccharides by Acinetobacter strains in a controlled fed-batch fermentation process using soap stock oil (SSO) as carbon source. Int J Biol Macromol. 1990 Apr;12(2):145–152. doi: 10.1016/0141-8130(90)90066-j. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Troller J. A. Effect of water activity on enterotoxin B production and growth of Staphylococcus aureus. Appl Microbiol. 1971 Mar;21(3):435–439. doi: 10.1128/am.21.3.435-439.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler U. K., Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979 Jun;138(3):663–670. doi: 10.1128/jb.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]