Abstract
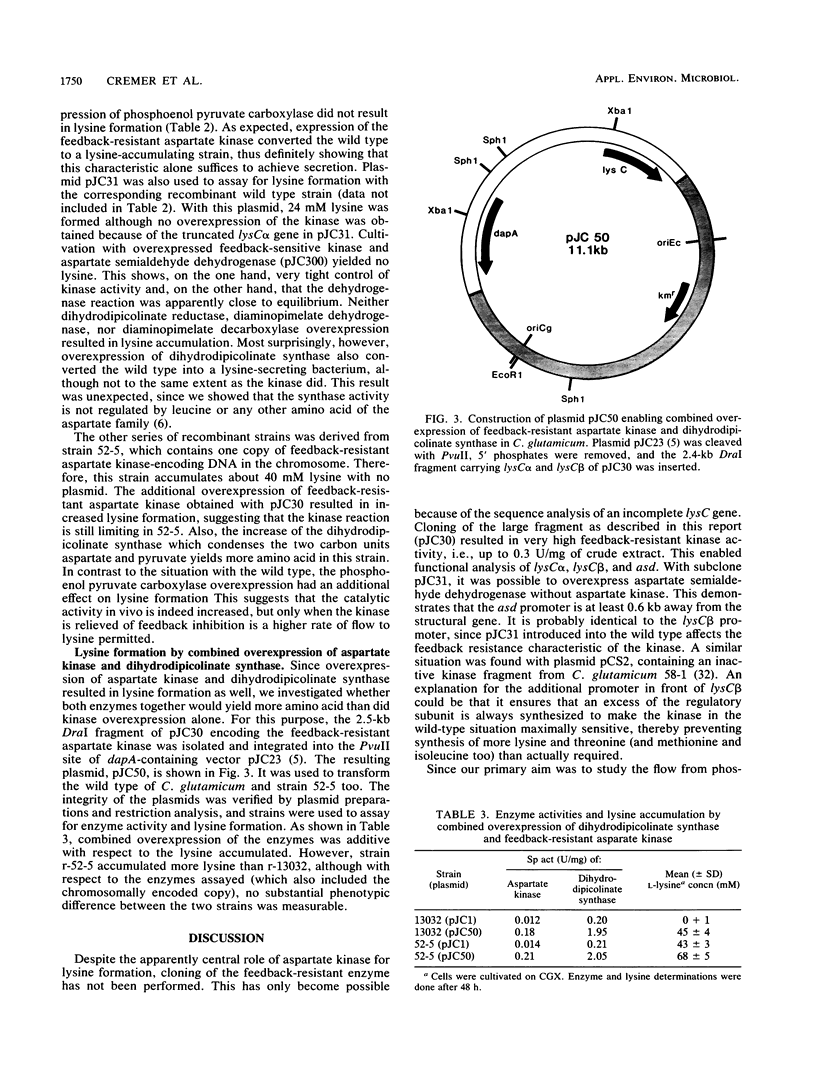
The gene cluster that codes for feedback-resistant aspartate kinase (lysCα and lysCβ) and aspartate semialdehyde dehydrogenase (asd) was cloned from a mutant strain of Corynebacterium glutamicum. Its functional analysis by subcloning, enzyme assays, and type of aspartate kinase regulation enabled the isolation of a fragment for separate expression of the feedback-resistant kinase without aspartate semialdehyde dehydrogenase expression. This was used together with other clones constructed (J. Cremer, L. Eggeling, and H. Sahm, Mol. Gen. Genet. 220:478-480, 1990) to overexpress individually each of the six genes that convert aspartate to lysine. Analysis of lysine formation revealed that overexpression of the feedback-resistant kinase alone suffices to achieve lysine formation (38 mM). Also, sole overexpression of wild-type dihydrodipicolinate synthase resulted in lysine formation but in a lower amount (11 mM). The other four enzymes had no effect on lysine secretion. With a plasmid overexpressing both relevant enzymes together, a further increase in lysine yield was obtained. This shows that of the six enzymes that convert aspartate to lysine the kinase and the synthase are responsible for flow control in the wild-type background and can be useful for construction of lysine-producing strains.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S., WRIGHT N. G. beta-Aspartokinase and beta-aspartyl phosphate. J Biol Chem. 1955 Mar;213(1):27–38. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Y., Hu F. M., Paulus H. Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem. 1987 Jun 25;262(18):8787–8798. [PubMed] [Google Scholar]
- Chen N. Y., Paulus H. Mechanism of expression of the overlapping genes of Bacillus subtilis aspartokinase II. J Biol Chem. 1988 Jul 5;263(19):9526–9532. [PubMed] [Google Scholar]
- Cremer J., Treptow C., Eggeling L., Sahm H. Regulation of enzymes of lysine biosynthesis in Corynebacterium glutamicum. J Gen Microbiol. 1988 Dec;134(12):3221–3229. doi: 10.1099/00221287-134-12-3221. [DOI] [PubMed] [Google Scholar]
- Eikmanns B. J., Follettie M. T., Griot M. U., Sinskey A. J. The phosphoenolpyruvate carboxylase gene of Corynebacterium glutamicum: molecular cloning, nucleotide sequence, and expression. Mol Gen Genet. 1989 Aug;218(2):330–339. doi: 10.1007/BF00331286. [DOI] [PubMed] [Google Scholar]
- Farkas W., Gilvarg C. The reduction step in diaminopimelic acid biosynthesis. J Biol Chem. 1965 Dec;240(12):4717–4722. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hoischen C., Krämer R. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol. 1990 Jun;172(6):3409–3416. doi: 10.1128/jb.172.6.3409-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino S., Mizukami T., Yamaguchi K., Katsumata R., Araki K. Nucleotide sequence of the meso-diaminopimelate D-dehydrogenase gene from Corynebacterium glutamicum. Nucleic Acids Res. 1987 May 11;15(9):3917–3917. doi: 10.1093/nar/15.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kalinowski J., Bachmann B., Thierbach G., Pühler A. Aspartokinase genes lysC alpha and lysC beta overlap and are adjacent to the aspartate beta-semialdehyde dehydrogenase gene asd in Corynebacterium glutamicum. Mol Gen Genet. 1990 Dec;224(3):317–324. doi: 10.1007/BF00262424. [DOI] [PubMed] [Google Scholar]
- LaRossa R. A., Van Dyk T. K., Smulski D. R. Toxic accumulation of alpha-ketobutyrate caused by inhibition of the branched-chain amino acid biosynthetic enzyme acetolactate synthase in Salmonella typhimurium. J Bacteriol. 1987 Apr;169(4):1372–1378. doi: 10.1128/jb.169.4.1372-1378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel E., Thierbach G., Eggeling L., Sahm H. Influence of increased aspartate availability on lysine formation by a recombinant strain of Corynebacterium glutamicum and utilization of fumarate. Appl Environ Microbiol. 1989 Mar;55(3):684–688. doi: 10.1128/aem.55.3.684-688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misono H., Togawa H., Yamamoto T., Soda K. Meso-alpha,epsilon-diaminopimelate D-dehydrogenase: distribution and the reaction product. J Bacteriol. 1979 Jan;137(1):22–27. doi: 10.1128/jb.137.1.22-27.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D., Paulus H. Properties and subunit structure of aspartokinase II from Bacillus subtilis VB217. J Biol Chem. 1977 Jul 10;252(13):4648–4651. [PubMed] [Google Scholar]
- O'Regan M., Thierbach G., Bachmann B., Villeval D., Lepage P., Viret J. F., Lemoine Y. Cloning and nucleotide sequence of the phosphoenolpyruvate carboxylase-coding gene of Corynebacterium glutamicum ATCC13032. Gene. 1989 Apr 30;77(2):237–251. doi: 10.1016/0378-1119(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Shiio I., Miyajima R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem. 1969 Jun;65(6):849–859. doi: 10.1093/oxfordjournals.jbchem.a129089. [DOI] [PubMed] [Google Scholar]
- Thierbach G., Kalinowski J., Bachmann B., Pühler A. Cloning of a DNA fragment from Corynebacterium glutamicum conferring aminoethyl cysteine resistance and feedback resistance to aspartokinase. Appl Microbiol Biotechnol. 1990 Jan;32(4):443–448. doi: 10.1007/BF00903780. [DOI] [PubMed] [Google Scholar]
- YUGARI Y., GILVARG C. Coordinate end-product inhibition in lysine synthesis in Escherichia coli. Biochim Biophys Acta. 1962 Aug 27;62:612–614. doi: 10.1016/0006-3002(62)90256-1. [DOI] [PubMed] [Google Scholar]
- Yamakura F., Ikeda Y., Kimura K., Sasakawa T. Partial purification and some properties of pyruvate-aspartic semialdehyde condensing enzyme from sporulating Bacillus subtilis. J Biochem. 1974 Sep;76(3):611–621. doi: 10.1093/oxfordjournals.jbchem.a130605. [DOI] [PubMed] [Google Scholar]
- Yeh P., Sicard A. M., Sinskey A. J. Nucleotide sequence of the lysA gene of Corynebacterium glutamicum and possible mechanisms for modulation of its expression. Mol Gen Genet. 1988 Apr;212(1):112–119. doi: 10.1007/BF00322452. [DOI] [PubMed] [Google Scholar]


