Abstract
Of the DNA bases, peroxynitrite (ONOO−) is most reactive toward 2′-deoxyguanosine (dGuo), but even more reactive with 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo), requiring a 1,000-fold excess of dGuo to provide 50% protection against the reaction with 8-oxodGuo. Therefore, it seems reasonable that 8-oxodGuo is a potentially important target in DNA and that the structures of the reaction products with ONOO− should be characterized. Using 3′,5′-di-O-Ac-8-oxodGuo as a model compound, the reaction products with ONOO− have been isolated and identified under simulated physiological reaction conditions (phosphate/bicarbonate buffer at pH 7.2). The major reaction product, II, is unstable and undergoes base-mediated hydrolysis to 2,5-diaminoimidazol-4-one, IIa, and 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-5-iminoimidazolidine-2,4-dione, IIb. The latter compound further hydrolyzes to 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)oxaluric acid, IIc. Other products include 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-2,4,6-trioxo-[1,3,5]triazinane-1-carboxamidine, I, which further hydrolyzes to 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)cyanuric acid, Ia. 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)parabanic acid, III, is a minor product that also may contribute to formation of IIc. The major products formed in these reactions are biologically uncharacterized but are similar to modified DNA bases that have been shown to be both premutagenic and blocks to DNA polymerization.
Peroxynitrite (ONOO−) is formed by the diffusion-limited combination of nitric oxide (NO⋅) and superoxide (O2⋅-) (1) and is a highly reactive molecule; it is stable in the anion form (pH = 12) but decays rapidly with a half-life of 1 s at physiologic pH in a process that involves protonation to form peroxynitrous acid (ONOOH, pKa = 6.8). It is proposed that the protonation of ONOO− results in a high-energy, reactive intermediate, ONOOH*, which can either decay to give nitrate or react with substrate (2, 3). In the presence of bicarbonate, the peroxynitrosocarbonate anion, (ONOOCO2−) is formed from ONOO− in a carbon dioxide catalyzed reaction (4–7). Although ONOOCO2− has a shorter lifetime than ONOO−, the former is still a potent oxidizing agent, with a redox potential in excess of + 1 V (4, 8).
An important source of ONOO− in vivo is the activated macrophage. This cell type has been implicated in causing tissue damage during acute and chronic inflammatory conditions by mechanisms that might involve ONOO− (9). Indeed, 3-nitrotyrosine, a putative marker of ONOO− formation, has been detected in people with acute lung injury (10, 11) and rheumatoid arthritis (12). In addition to reacting with lipids (13), proteins (14), and cellular thiols (15–19), ONOO− can cause DNA damage. The spectrum of DNA damage includes strand breakage (20–22) and both oxidation (22) and nitration (23) of bases.
Of the DNA nucleosides, 2′-deoxyguanosine (dGuo) is the most reactive toward ONOO−. Several reaction products are observed including the nitration product, 8-nitro-2′-deoxyguanosine (23); the addition product, 4,5-dihydro-5-hydroxy-4-(nitrosooxy)-2′-deoxyguanosine (24); and the oxidation products, 2,2-diamino-4-[(2-deoxy-β-d-erythro-pentofuranosyl)amino]-5-(2H)-oxazolone and 4-hydroxy-8-oxo-4,8-dihydro-2′-deoxyguanosine (25). Furthermore, 8-oxodGuo is more reactive toward ONOO− than is dGuo, requiring a 1,000-fold excess of dGuo to provide 50% protection of 8-oxodGuo from reaction with ONOO− (26). 8-oxodGuo can be present in cellular DNA at levels in the range of 4 to 11 in 107 bases (27). Further, given the preferential reactivity of this lesion toward ONOO−, we have hypothesized that knowledge of the structure of the lesions produced by reaction of 8-oxodGuo with ONOO− could be essential in understanding how ONOO− induces DNA damage and plays a role in mutagenesis.
Given the propensity for 8-oxodGuo to undergo further oxidation, we reasoned that this would be the most likely mode of reaction of 8-oxodGuo with ONOO−. There are several important models for the oxidation of 8-oxodGuo: (i) the alkaline potassium permanganate or iodine-mediated oxidation of uric acid; (ii) the electrochemical oxidation of 8-oxoGua; and (iii) the photooxidation of 8-oxodGuo. Alkaline oxidation of uric acid leads to the formation of allantoin and dehydroallantoin as major products (28). Electrochemical oxidation of 8-oxoGua results in formation of 2,5-diaminoimidazol-4-one (IIa) and 5-guanidinohydantoin (29). Photooxidation of 8-oxodGuo leads to the formation of 1,3,5-triazine-1(2H)-carboximidamide, 3-(2-deoxy-β-d-erythro-pentofuranosyl)tetrahydro-2,4,6-trioxo-(I) as the major product. This compound then undergoes base-mediated hydrolysis to yield 1-(2-deoxy-β-d-erythro-pentofuranosyl)cyanuric acid (Ia) (30).
Using these systems as models for the ONOO−-mediated oxidation of 8-oxodGuo, the major immediate products of this reaction have been identified.
Materials and Methods
Materials.
Cyanuric acid, parabanic acid, potassium oxonate, and 2-amino-6,8-dihydroxypurine (8-oxoGua) were obtained from Aldrich (Milwaukee, WI). 8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo) was either obtained from Sigma or synthesized as described by Kasai and Nishimura (31). Potassium permanganate (Fisher Scientific), sodium borohydride (NaBH4) (American Bioanalytical, Natick, MA), and acetic anhydride (Mallinckrodt) were analytical grade. Silylation grade pyridine was obtained from Pierce, and bis(trimethylsilyl)trifluoro-acetamide was from Supelco. Peroxynitrite was synthesized by the ozonation of an aqueous alkaline solution of sodium azide (32). All solvents were HPLC grade.
Instrumentation.
UV/Vis measurements were made by using an HP8452 Diode Array Spectrophotometer (Hewlett–Packard). 1H-NMR spectra were recorded at 300 or 500 MHz and 13C-NMR spectra (proton decoupled) at 75 or 125 MHz on Unity 300 and Inova 500 spectrometers, respectively. HPLC was performed by using an HP1090 pump equipped with a 1090 Diode Array Detector (Hewlett–Packard). Electrospray ionization (ESI)-MS and tandem MS (ESI-MS/MS) experiments were carried out by using either an HP 5989B (Hewlett–Packard) or TSQ 7000 (Finnigan, San Jose, CA) mass spectrometer, respectively. High-resolution MS (HRMS) experiments were done in ESI mode on an APEX II Fourier transform MS (Bruker, Billerica, MA). All ESI-MS and ESI-MS/MS spectra were obtained in negative ion mode by using a spraying solution with the composition 49:40:10:1 water/methanol/acetonitrile/ammonium hydroxide or 78:20:2 water/2-propanol/ammonium hydroxide. In exchange experiments, D2O was substituted for H2O. GC/MS analyses were carried out on a HP 5989A mass spectrometer (Hewlett–Packard).
3′,5′-Di-O-Ac-8-oxodGuo Synthesis and Purification.
3′,5′-Di-O-Ac-8-oxodGuo was prepared by heating a suspension of 8-oxodGuo (1 mg) in pyridine (100 μl) and acetic anhydride (60 μl) at 70°C for 30 min. Unreacted acetic anhydride was quenched by addition of methanol (250 μl), and the mixture was dried in vacuo. The residue was dissolved in double-distilled water and purified by semipreparative HPLC on a 250 × 10 mm, 7 μm RP-300 column (Alltech Associates). Solvents A and B were 50 mM ammonium acetate, pH 5.5 and acetonitrile, respectively. Isocratic elution with 5% B for 5 min was followed by a gradient from 5% B to 22% B in 18 min. After holding at 22% B for 4 min, the column was ramped from 22% B to 5% B in 3 min. A flow rate of 2.5 ml/min was used, and products were monitored simultaneously at 225 nm and 260 nm. The desired 3′,5′-di-O-Ac-8-oxodGuo eluted at 19.8 min under these conditions. Fractions containing the desired product were collected, pooled, and lyophilized. Excess ammonium acetate was removed by taking up the residue in double-distilled water and lyophilizing several times. 1H-NMR (DMSO-d6, δ): 10.84 ppm (s, 1H, NH); 10.71 ppm (s, 1H, NH); 6.49 ppm (s, 2H, NH2); 6.02 (m, 1H, H1′); 5.35 ppm (m, 1H, H3′); 4.32 ppm (m, 1H, H4′); 4.09 ppm (m, 2H, H5"); 2.23 ppm (m, 1H, H2′); 2.09 (m, 6H, 2 x CH3); UV/Vis (0.1 M HCl): λmax = 248 nm, 292 nm; ESI-MS (negative ions) m/z 366 [M-H]−.
Synthesis of 5-Azauracil and (2-Imino-5-Oxo-Imidazolidin-4-yl)Urea.
5-Azauracil was synthesized by the decarboxylation of potassium oxonate as described by Pike (33). The pure compound was obtained by recrystallization of the crude product from methanol. 1H-NMR (DMSO-d6, δ): 11.32 ppm (s, 1H, NH), 8.14 ppm (s, 1H, CH); 13C-NMR (DMSO-d6, δ): 157.18 ppm (C2/C4), 66.71 (C6); ESI-MS (negative ions) m/z 112 [M-H]−.
(2-Imino-5-oxo-imidazolidin-4-yl)urea was prepared by the alkaline potassium permanganate oxidation of 8-oxoGua by analogy with the preparation of allantoin from uric acid (34). The analytical sample was obtained after recrystallization of the crude product from water. 1H-NMR (DMSO-d6, δ): 7.92 ppm (s, 1H, 3-NH), 7.68 ppm (s, 1H, 2-NH), 6.93 ppm (s, 1H, 1-NH), 6.7 ppm (d, 1H, J = 7.8 Hz, 6-NH), 5.69 ppm (s, 2H, 8-NH2), 5.08 ppm (d, 1H, J = 8.1 Hz, 4-CH); 13C-NMR (DMSO- d6, δ): 185.99 ppm (C2), 171.94 ppm (C5), 158.36 ppm (C7), 64.34 (C4); HRMS calculated for C4H6N5O2 [M-H]− 156.0522, found 156.0525.
Reaction of 3′,5′-Di-O-Ac-8-oxodGuo with ONOO-.
Reactions of 3′,5′-di-O-Ac-8-oxodGuo with ONOO− were carried out in 150 mM KH2PO4, 25 mM Na2CO3, pH 7.2 buffer. The desired concentrations of 3′,5′-di-O-Ac-8-oxodGuo and ONOO− were determined spectrophotometrically by using the extinction coefficients 13.5 mM−1⋅cm−1 (λ = 248 nm, 0.1 M HCl) (35) and 1,670 M−1⋅cm−1 (λ = 302 nm, 0.1 M NaOH) (36), respectively. Reactions were performed by adding the desired amount of 3′,5′-di-O-Ac-8-oxodGuo to a buffered solution in an Eppendorf tube (1.5 ml). ONOO− was placed as a droplet on the underside of the lid. After carefully closing, the tube was vigorously vortexed for 1 min to effect complete reaction.
Separation of 3′,5′-Di-O-Ac-8-oxodGuo/ONOO- Reaction Products.
After reacting 100 μM 3′,5′-di-O-Ac-8-oxodGuo with 500 μM ONOO−, products were separated by HPLC using a 250 × 4.6 mm, 5 μm LC-18-DB column (Supelco). The mobile phases used were 50 mM ammonium acetate and acetonitrile. HPLC conditions used were: isocratic 5% B for 10 min; 5% B to 27.4% B in 20 min; 27.4% B to 5% B in 5 min. The flow rate was 1 ml/min, and products were monitored simultaneously at 230 nm and 260 nm.
Reduction of Compound II.
For analytical studies, a 100 μM 3′,5′-di-O-Ac-8-oxodGuo solution was treated with 500 μM ONOO− (final total volume = 1 ml). Treating either the HPLC-purified product or the crude 3′,5′-di-O-Ac-8-oxodGuo/ONOO− reaction mixture with NaBH4 effected reduction of II. The fraction containing purified II (volume = 350–400 μl) or the crude reaction mixture (volume = 1 ml) was treated with 5 μl of an aqueous 1 M NaBH4 stock solution. The mixtures were incubated at room temperature for 5–10 min and then analyzed by HPLC using the same conditions for purifying the 3′,5′-di-O-Ac-8-oxodGuo/ONOO− reaction products. Reduction of II was complete and yielded two products, IIred (λmax = 229 nm, Mr = 357) and II′red (λmax = 227 nm, Mr = 357), which had retention times of 18.1 and 18.9 min, respectively. In preparative studies, 0.084 mmol (30 mg) of 3′,5′-di-O-Ac-8-oxodGuo was treated with 0.50 mmol ONOO− in 150 mM KH2PO4, 25 mM Na2CO3, pH 7.2 (80 ml), and 0.80 mmol NaBH4 added to reduce the products formed. The reaction mixture was concentrated by rotary evaporation and purified by semipreparative HPLC on a Nucleosil C18 250 × 10 mm, 5 μm column (Alltech Associates) with the same gradient and solvents used in the analytical studies and a flow rate = 3.0 ml/min. Under these conditions, IIred/II′red eluted as a single peak. The product was desalted on a C18 Mega Bond Elut column (Varian), eluted with methanol and dried in vacuo. 1H-NMR (DMSO-d6, δ): (diastereomer 1) 7.44 ppm (s, br, 2H); 5.81 ppm (dd, J = 5.5 Hz, 10 Hz, 1H, H1′); 5.30 ppm (s, 1H, H4); 5.06 ppm (m, 1H, H3′); 4.15–3.90 ppm (m, 3H, H4′, H5′, H5"); 2.30 ppm* (m, 1H, H2′); 2.18 ppm* (m, 1H, H2"); 2.07 ppm (m, 6H, 2 x CH3); (diastereomer 2) 7.44 ppm (s, br, 2H); 5.66 ppm (dd, J = 6 Hz, 9 Hz, 1H, H1′); 5.19 ppm (s, 1H, H4); 5.00 ppm (m, 1H, H3′); 4.15–3.90 ppm (m, 3H, H4′, H5′, H5"); 2.30 ppm* (m, 1H, H2′); 2.18 ppm* (m, 1H, H2"); 2.05 ppm (m, 6H, 2 x CH3); HRMS calculated for C13H18N5O7 [M-H]− 356.1205, found 356.1207. ∗ indicates recorded in D2O. Considerable overlap of the H2′ and H2′′ resonances for the respective diastereomers is observed.
Hydrolysis of II.
A 100-μM 3′,5′-di-O-Ac-8-oxodGuo solution was treated with 750 μM ONOO− and II isolated by analytical HPLC as described before, except that 60 mM KH2PO4, pH 7.4 was used as the aqueous mobile phase. Ten microliters of concentrated ammonium hydroxide was added to the fraction containing II (520 μl), and the mixture was incubated at room temperature for 1.5 hr. This hydrolysis mixture was analyzed by HPLC using the same conditions used to isolate II. Hydrolysis of II yielded IIa (λmax = 249 nm and 313 nm; Mr = 112), IIb (λmax = 225 nm, shoulder 260–310 nm; Mr = 313), and IIc (λmax = 213 nm; Mr = 332), which eluted at 3.3, 26.2, and 21 min, respectively.
Preparation of 2,3,5-Tri-O-Ac-β-d-erythro-Pentofuranosyl Derivative of IIc (IIc′).
The starting material, 2′,3′,5′-tri-O-Ac-8-oxoGuo, was prepared according to the procedure by Ikehara et al. (37), except that the 8-BrGuo was acetylated by using standard procedures (38). IIc′ then was prepared by reacting 2′,3′,5′-tri-O-Ac-8-oxoGuo (0.047 mmol, 20 mg) dissolved in 150 mM KH2PO4, 25 mM Na2CO3, pH 7.2 buffer (10 ml) with ONOO− (0.141 mmol, 1.68 ml). Reactions were initiated by forcefully pipetting the ONOO− into the vigorously stirred nucleoside solution. The product corresponding to II, (II′), was purified by semipreparative HPLC on a Nucleosil C18 250 × 10 mm, 5-μm column (Alltech Associates) by using 10 mM KH2PO4, pH 7.4 (solvent A) and acetonitrile (solvent B) as mobile phases. HPLC elution conditions used were: isocratic, 5% B for 10 min; 5% to 40% B in 35 min; 40% to 5% B in 5 min. The flow rate was 3.5 ml/min, and products were monitored simultaneously at 225 nm and 260 nm. II′ eluted at 32 min. Fractions containing II′ were pooled, dried, and taken up in 1.5 ml of double-distilled water and left at room temperature for 30 hr. Complete hydrolysis to IIc′ was confirmed by HPLC before the mixture was desalted on a C18 Mega Bond Elut column (Varian), eluted with methanol, and dried in vacuo. 1H-NMR (DMSO-d6, δ): 9.81 ppm (s, 1H, COOH); 8.87 ppm (d, J = 9.3 Hz, 1H, NH); 7.04 (s, 1H, NH); 5.51 ppm (m, 1H, J = 5.1 Hz, 9.3 Hz, H1′); 5.22 ppm (m, 2H, H2′ and H3′); 4.23 ppm (m, 1H, H4′), 4.11 ppm (m, 2H, H5′ and H5"); 2.07 ppm (m, 9H, 3 x CH3); UV (H2O): λmax = 213 nm; ESI-MS (negative ions): m/z 390 [M-H]−.
Preparation of Reaction Products for GC/MS.
Ten 1-ml reactions of 100 μM 3′,5′-di-O-Ac-8-oxodGuo with 750 μM ONOO− were combined and dried in vacuo. The residue was taken up in double-distilled water (1 ml), and I, II, and III were purified by analytical HPLC using 50 mM ammonium acetate and acetonitrile as mobile phases. Isolated II was base-hydrolyzed to give IIa and IIb as described above. IIred and II′red were HPLC-purified from 10 separate reactions of 100 μM 3′,5′-di-O-Ac-8-oxodGuo with 750 μM ONOO−, with NaBH4 (5 μl of a 1 M stock) added to effect reduction of II. The HPLC fractions containing I, IIb, III, IIred, and II′red were dried in vacuo, and the residues were taken up in 6 M HCl (75 μl), transferred to silinized reacti-vials (0.5 ml), and heated at 100°C for 5 min to remove the 3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl moiety. These solutions then were cooled to room temperature and dried in vacuo. IIa, which lacks the 3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl moiety, was not subjected to acid hydrolysis. Hence, the HPLC fraction containing IIa was dried in vacuo and subsequently derivatized as described below. Samples were derivatized for GC/MS analysis using a 1:1 mixture of bis(trimethylsilyl)trifluoro-acetamide in acetonitrile (100 μl). The aglycons of IIb and III were derivatized by heating at 130°C for 10 min. The aglycons of I, IIa, IIred, and II′red were derivatized similarly, except that these samples were heated for 20 min. All samples were cooled to room temperature, and 5–10 μl was used per GC/MS analysis.
Preparation of Authentic Standards for GC/MS.
One hundred micrograms each of 5-azauracil, cyanuric acid, (2-imino-5-oxo-imidazolidin-4-yl)urea, and parabanic acid were transferred in aqueous solution to silinized reacti-vials. The solutions were dried in vacuo, and the residues were taken up in 100 μl of a 1:1 bis(trimethylsilyl)trifluoro-acetamide/acetonitrile mixture. Heating the respective solutions at 130°C for 10 min effected derivatization of 5-azauracil and parabanic acid. Cyanuric acid and (2-imino-5-oxo-imidazolidin-4-yl)urea were derivatized similarly, except that these solutions were heated for 20 min. Derivatized samples were diluted 10-fold with hexane and 1–5 μl was injected per analysis.
GC/MS Analyses.
All analyses were performed by using a 12.5 m × 0.22 mm × 0.33 μm film thickness HP-1 column (Hewlett–Packard). Either electron ionization or positive ion chemical ionization (PICI) was used. In PICI studies, methane was used as the bath gas. The injector and inlet temperatures were set at 250°C and the source temperature at 200°C. The quadrupole temperature was kept at 100°C. An electron ionization energy of 70 eV was used in all studies. Helium was used as the carrier gas, and the column head pressure was set at 6 psi. In all studies of the derivatized aglycons and authentic standards, the column temperature was ramped from 60°C at 0 min to 250°C at a rate of 20°C/min and then held constant at 250°C for 5 min. All injections were made in splitless mode.
Results and Discussion
The treatment of 3′,5′-di-O-Ac-8-oxodGuo with ONOO− followed by immediate HPLC analysis reveals the formation of four additional compounds. The first compound, I (λmax = 216 nm, Mr = 371), eluted at 23 min. The second compound, II (λmax = 236 nm; shoulder 275–350 nm; Mr = 355), eluted at 24.5 min, and the third compound, III (λmax = 220 nm and 280 nm; Mr = 314), eluted at 24.9 min. The fourth compound is not presently characterized and will not be discussed further. Over a range of ONOO− concentrations, II is the major product of the reaction of 3′,5′-di-O-Ac-8-oxodGuo with ONOO− whereas I and III are formed in lower but similar amounts. These three compounds have been studied in detail and structurally characterized.
Characterization of II.
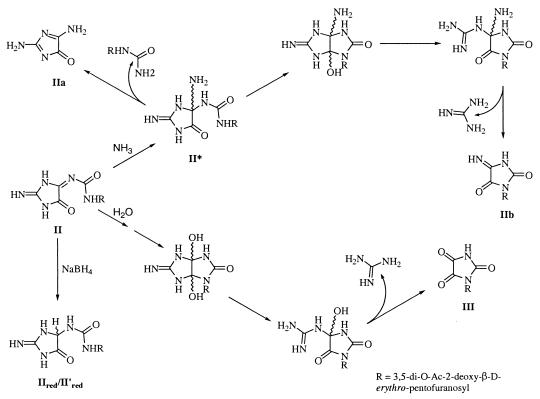
Direct structural studies of II were impaired because of its inherent instability. However, HRMS revealed that the molecular formula for this compound is C13H17N5O7 (calculated exact mass: 355.1128; found: 355.1125). II can be reduced by NaBH4 in 100% yield to give equal amounts of two stable compounds, IIred and II′red, both with molecular formulae C13H19N5O7 (calculated exact mass: 357.1284; found: 357.1286). By characterizing the stable reduction products along with the hydrolysis products, it was possible to propose that the intermediate II is most likely 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3-(2-imino-5-oxo-imidazolidin-4-ylidene)urea (Fig. 1).
Figure 1.
Structure proposed for II, along with its reduction and hydrolytic products.
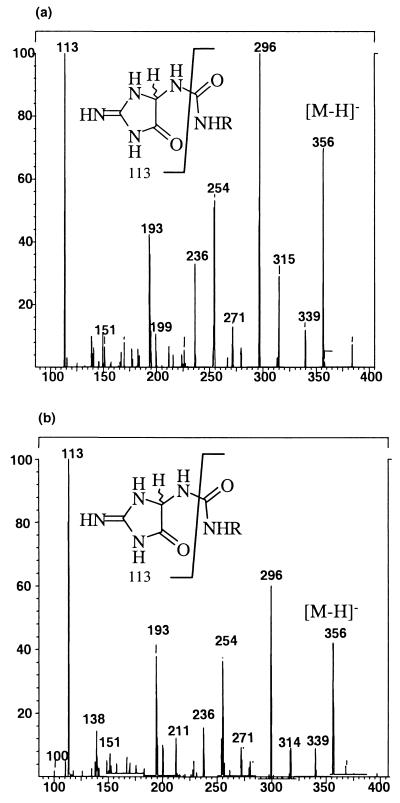
Several lines of evidence support this conclusion. The reduction products IIred and II′red were acid-hydrolyzed to yield the aglycons, and the trimethylsilyl derivatives of the aglycons were prepared and analyzed by GC/MS. Both IIred and II′red released a single aglycon that had the same retention time and fragmentation pattern as that of authentic (2-imino-5-oxo-imidazolidin-4-yl)urea (Table 1). This finding suggested that IIred and II′red constituted a diastereomeric pair. The 1H-NMR spectra of IIred and II′red were essentially identical, a finding consistent with these compounds being diastereomers. Additionally, ESI-MS/MS experiments revealed that these compounds fragmented identically, yielding a major fragment in both cases with m/z = 113, which corresponds to the 5-amino-2-imino-imidazolidin-4-one portion of IIred/II′red (Fig. 2). Taken together, these data indicate that IIred/II′red are diastereomers of 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3-(2-imino-5-oxo-imidazolidin-4-yl)urea.
Table 1.
GC/MS data for authentic standards
| Compound | Retention time, min | Ions |
|---|---|---|
| 5-Azauracil, IIb | 5.3 | 257 (M+, 59%); 242 (54%); 143 (68%); 127 (31%); 100 (base peak) |
| Cyanuric acid, Ia | 6.8 | 345 (base peak, M+); 330 (36%); 147 (44%); 100 (40%) |
| (2-Imino-5-oxo-imidazolidin-4-y)urea, IIred | 9.0 | 517 (base peak, M+); 502 (22%); 428 (78%); 356 (33%) |
| Parabanic acid, III | 5.8 | 258 (M+, 25%); 243 (76%); 215 (15%); 188 (5%); 100 (base peak) |
The aglycons of the OONO−-reaction products of 3′,5′-di-O-Ac-8-oxodGuo are shown in bold type next to the authentic standard that gave an identical retention time and fragmentation pattern.
Figure 2.
Negative ion ESI-MS/MS spector for (a) IIred and (b) II′red. Both compounds fragment identically, a finding consistent with their assignment as the diastereomers of 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3-(2-imino-5-oxo-imidazolidin-4-yl)urea.
The H1′ proton of the 3,5-di-O-Ac-β-d-erythro-pentofuranosyl diastereomers of IIred appear as a doublet of doublets in DMSO-d6 (J = 5.8 Hz and 9.5 Hz) as expected from coupling between the H1′ and the nonidentical H2′ and H2′′ protons. The expected coupling between the H1′ and the 1-NH protons was not observed. This finding likely arises because of rapid exchange of the 1-NH proton with trace amounts of water present in the solution. However, in 1H-NMR experiments carried out on the 2,3,5-tri-O-Ac-β-d-erythro-pentofuranosyl diastereomers of IIred in DMSO-d6, the H1′ appeared as a doublet of doublets, indicating coupling of the H1′ to both the H2′ (J = 6.3 Hz) and the 1-NH proton (J = 12 Hz). On the other hand, when 1H-NMR spectra were obtained in D2O, the H1′ appeared as a doublet (J = 6.5 Hz). This indicates exchange of the 1-NH proton for deuterium with concomitant loss of coupling between the H1′ and 1-NH protons. These findings further support the structural assignment of IIred as 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3-(2-imino-5-oxo-imidazolidin-4-yl)urea.
The hydrolysis products of II also were characterized to gain further insight into its structure. Ammonium hydroxide-mediated hydrolysis yielded three compounds, IIa, IIb, and IIc. IIa (Mr = 112) has UV maxima at 249 nm and 313 nm and an HPLC retention time of 3.3 min. The dramatically shortened retention time relative to all of the primary reaction products along with the molecular weight of IIa indicated that the 3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl moiety, Mr = 201) was not attached to IIa. The UV spectrum of IIa was found to be identical to that reported for 2-amino-5-[(2-deoxy-β-d-erythro-pentofuranosyl)amino]-4H-imidazol-4-one (39). Furthermore, the mass spectrum of IIa using GC/MS in electron ionization mode revealed ions at m/z = 328, 313, 285, and 198, a spectrum identical to that reported for 2,5-diaminoimidazol-4-one (29). Hence, IIa is identified as 2,5-diaminoimidazol-4-one.
The second hydrolysis product, IIb (Mr = 313), has λmax = 225 nm and a shoulder from 260 to 310 nm and an HPLC retention time of 26.2 min. HRMS indicated that this compound has a molecular formula C12H15N3O7 (calculated exact mass: 313.0910; found: 313.0908) and hence, a base fragment with molecular formula, C3H2N3O2. Negative ion ESI-MS analysis of IIb performed by using H2O and D2O in the spray solvent resulted in [M-H]− ions at m/z = 312 and 313, respectively. This result indicated that IIb has two exchangeable protons, because in addition to having one deuteron in the observed ion, another deuteron was lost to form the observed negative ion. These exchangeable protons must be present in the base fragment because the 3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl moiety has no exchangeable protons. Therefore, IIb is identified as 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-5-iminoimidazolidine-2,4-dione. Further, as has been reported previously for substituted 5-iminoimidazolidine-2,4-dione compounds, strong acid or base treatment will cause ring expansion to yield s-triazine structures, including oxonic acid and 5-azauracil, by mechanisms that are not clearly understood (28). Indeed, acid hydrolysis of IIb led to release of an aglycon that was identified as 5-azauracil based on an identical GC/MS retention time and fragmentation pattern as authentic 5-azauracil (Table 1), thus further indirectly supporting the proposed structure for IIb.
Importantly, IIb is unstable and undergoes hydrolysis to yield IIc as a stable end product. IIc, as determined by negative ion ESI-MS, has a Mr = 332 and three exchangeable protons. To facilitate 1H-NMR studies aimed at identifying the aglycon of IIc, the 2,3,5-tri-O-Ac-β-d-erythro-pentofuranosyl analog (IIc′), was used. IIc′ was used because it could be prepared from the parent compound, 2′,3′,5′-tri-O-Ac-8-oxoGuo, which is easily accessible synthetically and in high yield. We verified that IIc and IIc′ had identical UV/Vis spectra and that the molecular weight of IIc′ (Mr = 390) was the expected 58 atomic mass units greater than that of IIc (Mr = 332).
The aglycon of IIc′ (and hence that of IIc) was identified as oxaluric acid. Three nonsugar protons were observed at δ = 9.81 ppm (s, 1H, COOH), 8.87 ppm (d, 1H, J = 9.3 Hz, 3-NH), and 7.04 ppm (s, 1H, 1-NH). All three protons are D2O exchangeable, in agreement with results determined by ESI-MS. The δ = 8.87 ppm peak is coupled to the sugar H1′ (δ = 5.51 ppm, 1H, J = 5.1 Hz, 9.3 Hz). Exchange of the 3-NH proton when D2O is added results in H1′ appearing as a doublet with J = 5.1 Hz, which confirms that the sugar is attached at the N3 position. Therefore, it is concluded that IIc is 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)oxaluric acid. Indeed, if substitution were at the N1 position, both amide protons would be identical (3-NH2) and no coupling between the 3-NH2 and H1′ would be expected.
Any structure proposed for II must account for the fact that reduction will yield the diastereomeric pair, IIred and II′red and the observed hydrolysis products, IIa, IIb, and IIc. As shown in Fig. 1, reduction of II will directly yield the diastereomers IIred and II′red. Ammonium hydroxide-mediated ammonolysis or hydrolysis of II likely proceeds via nucleophilic attack at C4. Ammonolysis with elimination of (3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)urea leads to formation of IIa. Alternatively, ring closure to yield intermediate II* followed by elimination of guanidine yields IIb (Fig. 1), which ultimately hydrolyses to IIc.
Characterization of I and III.
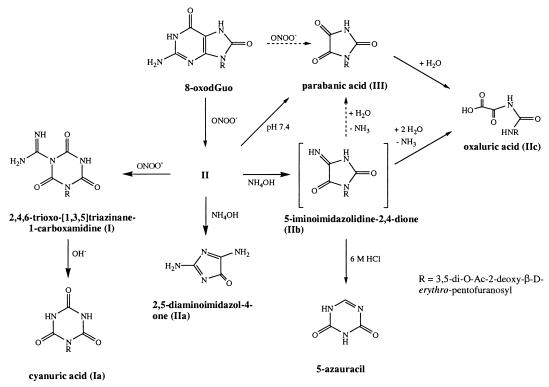
I and III were acid-hydrolyzed to obtain the aglycons. The trimethylsilyl derivative of the released aglycons was prepared and analyzed by GC/MS. The derivatized aglycons from I and III were found to have retention times and fragmentation patterns identical to those of authentic cyanuric acid and parabanic acid, respectively (Table 1). The molecular weight calculated for the 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)parabanic acid matched that observed by ESI-MS. Further, the UV spectrum of III (λmax = 220 nm and 280 nm) matched that of the authentic parabanic acid. Therefore, III was identified as 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)parabanic acid. This compound also is formed as a hydrolysis product of II upon incubation in 150 mM KH2PO4, pH 7.4 buffer (Fig. 1) and also will yield IIc under the same conditions (Fig. 3).
Figure 3.
Summary of the peroxynitrite reaction products of 3′,5′-di-O-Ac-8-oxodGuo.
In the case of I, the calculated molecular weight of the 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)cyanuric acid (Ia) derivative (Mr = 329) was 42 atomic mass units lower than the molecular weight determined for I (Mr = 371) by ESI-MS. However, ESI-MS/MS of I revealed ions with m/z = 370, 328, and 285. The m/z = 370 ion corresponds to the [M-H]− ion of I. The m/z = 328 ion is the [M-CH3N2+H]− ion and corresponds to the ion expected for Ia. The m/z = 285 ion arises because of neutral loss of an [HN=C=O] fragment from the parent ion of Ia. These data indicate that Ia can arise from I either by hydrolysis or under ESI-MS/MS conditions and taken together led us to identify I and Ia as 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-2,4,6-trioxo-[1,3,5]triazinane-1-carboxamidine and 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)cyanuric acid, respectively, both of which also are formed during the reaction of 8-oxodGuo with singlet oxygen (30).
Conclusion
Fig. 3 summarizes both the immediate and subsequent hydrolysis products formed by the reaction of 3′,5′-di-O-Ac-8-oxodGuo with ONOO−. II is the major product, whereas I and III are formed in lower amounts. I, II, and III are present in the reaction mixture immediately after ONOO− treatment. Interestingly, formation of II plateaus in the presence of unreacted 3′,5′-di-O-Ac-8-oxodGuo, suggesting that it might be reacting further with ONOO−. Indeed, treatment of isolated II with ONOO− led to formation of I, indicating that I can arise as a further oxidation product of II. Both I and III do not react further with ONOO− and hence are terminal oxidation products of the ONOO−-mediated oxidation of 3′,5′-di-O-Ac-8-oxodGuo.
All three products are hydrolytically labile. In 150 mM KH2PO4, 25 mM Na2CO3, pH 7.2 and at ambient temperature, II has a half-life of 5 hr. This compound is therefore relatively more labile than I whose half-life previously was determined to be days (30). However, IIc is quite stable and little degradation occurs even after days of incubation at room temperature in pH 7.2 buffer. We have shown that I can arise from the further reaction of II with ONOO−. At present, however, it is not possible to definitively say whether III arises solely as a hydrolysis product of II or whether it is also a direct product of the reaction of 3′,5′-di-O-Ac-8-oxodGuo with ONOO−.
The instability of II has impaired our efforts to rigorously characterize this compound. However, the reduction products, in particular, are highly informative about its structure. Reduction leads to formation of the diastereomers, IIred and II′red, which is most consistent with the assignment of II as 1-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3-(2-imino-5-oxo-imidazolidin-4-ylidene)urea. Presently, we cannot completely exclude the possibility that II is the ring-closed structure, 3-(3,5-di-O-Ac-2-deoxy-β-d-erythro-pentofuranosyl)-3a-hydroxy-5-imino-3,3a,4,5-tetrahydro-1H-imidazo[4,5-d]imidazol-2-one. The bicyclic and the ring-opened structures are not mutually exclusive possibilities, however, as the former structure is highly strained and may be in equilibrium with the ring-opened structure.
In conclusion, we have identified the major immediate and hydrolysis products of the reaction of 3′,5′-di-O-Ac-8-oxodGuo with ONOO−. Under our experimental conditions, II is the major product at the low ONOO− treatment levels. This is important when considering the reaction of 8-oxoGua lesions in DNA with ONOO−. Our experiments indicate that these lesions also are formed in oligonucleotides containing an 8-oxoGua residue (40). Understanding the impact of the reaction of 8-oxoGua in DNA with ONOO− will require studying specifically the formation and fate of II in DNA. The half-life of II at physiologic pH (5 hr) indicates that within 2 days of formation, it is completely hydrolyzed to its stable hydrolysis product, IIc. Lesions bearing remarkable structural resemblance to IIc, for example the glycoside of β-ureidoisobutyric acid, have been shown to be premutagenic and to function as potent blocks to DNA replication (41).
Acknowledgments
We thank Gary Kruppa at Bruker for providing access to an APEX II Fourier transform MS and Christian Berg and Paul Speir for their assistance in acquiring HRMS data. Special thanks to Dr. Richard Loeppky for critically reading this manuscript and sharing his chemical and mechanistic insights. This work was supported by National Institutes of Health Grants 5-F31-HG00144–05 and CA26731.
Abbreviations
- ESI
electrospray ionization
- MS/MS
tandem MS
- HRMS
high-resolution MS
References
- 1.Huie R E, Padmaja S. Free Radical Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 2.Pryor W A, Squadrito G L. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 3.Koppenol W H, Moreno J J, Pryor W A, Ischiropoulos H, Beckman J S. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 4.Lymar S V, Jiang Q, Hurst J K. Biochemistry. 1996;35:7855–7861. doi: 10.1021/bi960331h. [DOI] [PubMed] [Google Scholar]
- 5.Lymar S V, Hurst J K. Chem Res Toxicol. 1996;9:845–850. doi: 10.1021/tx960046z. [DOI] [PubMed] [Google Scholar]
- 6.Pryor W A, Lemercier J, Zhang H, Uppu R M, Squadrito G L. Free Radical Biol Med. 1997;23:331–338. doi: 10.1016/s0891-5849(97)00121-4. [DOI] [PubMed] [Google Scholar]
- 7.Uppu R M, Squadrito G L, Pryor W A. Arch Biochem Biophys. 1996;327:335–343. doi: 10.1006/abbi.1996.0131. [DOI] [PubMed] [Google Scholar]
- 8.Lymar S V, Hurst J K. Inorg Chem. 1998;37:294–301. [Google Scholar]
- 9.Ischiropoulos H, Zhu L, Beckman J S. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 10.Haddad I, Pataki G, Hu P, Galliani C, Beckman J S, Matalon S. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooy N W, Royall J A, Ye Y Z, Kelly D R, Beckman J S. Am J Respir Crit Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 12.Kaur H, Halliwell B. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 13.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 14.Ischiropoulos H, Al-Mehdi A B. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 15.Radi R, Beckman J S, Bush K M, Freeman B A. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 16.Moreno J J, Pryor W A. Chem Res Toxicol. 1992;5:425–431. doi: 10.1021/tx00027a017. [DOI] [PubMed] [Google Scholar]
- 17.Gatti R M, Radi R, Augusto O. FEBS Lett. 1994;348:287–290. doi: 10.1016/0014-5793(94)00625-3. [DOI] [PubMed] [Google Scholar]
- 18.Mohr S, Stamler J S, Brune B. FEBS Lett. 1994;348:223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- 19.Crow J P, Beckman J S, McCord J M. Biochemistry. 1995;34:3544–3552. doi: 10.1021/bi00011a008. [DOI] [PubMed] [Google Scholar]
- 20.Salgo M G, Stone K, Squadrito G L, Battista J R, Pryor W A. Biochem Biophys Res Commun. 1995;210:1025–1030. doi: 10.1006/bbrc.1995.1759. [DOI] [PubMed] [Google Scholar]
- 21.Yermilov V, Yoshie Y, Rubio J, Ohshima H. FEBS Lett. 1996;399:67–70. doi: 10.1016/s0014-5793(96)01288-4. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy L J, Moore K, Jr, Caulfield J L, Tannenbaum S R, Dedon P C. Chem Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- 23.Yermilov V, Rubio J, Becchi M, Friesen M D, Pignatelli B, Ohshima H. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 24.Douki T, Cadet J, Ames B N. Chem Res Toxicol. 1996;9:3–7. doi: 10.1021/tx950126n. [DOI] [PubMed] [Google Scholar]
- 25.Douki T, Cadet J. Free Radical Res. 1996;24:369–380. doi: 10.3109/10715769609088035. [DOI] [PubMed] [Google Scholar]
- 26.Uppu R M, Cueto R, Squadrito G L, Salgo M G, Pryor W A. Free Radical Biol Med. 1996;21:407–411. doi: 10.1016/0891-5849(96)00220-1. [DOI] [PubMed] [Google Scholar]
- 27.Helbock H J, Beckman K B, Shigenaga M K, Walter P B, Woodall A A, Yeo H C, Ames B N. Proc Natl Acad Sci USA. 1998;95:288–293. doi: 10.1073/pnas.95.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poje M, Sokolic-Maravic L. Tetrahedron. 1986;42:747–751. [Google Scholar]
- 29.Goyal R N, Dryhurst G. J Electroanal Chem. 1982;135:75–91. [Google Scholar]
- 30.Raoul S, Cadet J. J Am Chem Soc. 1996;118:1892–1898. [Google Scholar]
- 31.Kasai H, Nishimura S. Nucleic Acids Res. 1984;12:2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryor W A, Cueto R, Jin X, Koppenol W H, Ngu-Schwemlein M, Squadrito G L, Uppu P L, Uppu R M. Free Radical Biol Med. 1995;18:75–83. doi: 10.1016/0891-5849(94)00105-s. [DOI] [PubMed] [Google Scholar]
- 33.Pike R K. Org Magnetic Resonance. 1976;8:224–225. [Google Scholar]
- 34.Poje M, Sokolic-Maravic L. Tetrahedron. 1988;44:6723–6728. [Google Scholar]
- 35.Lin T, Cheng J, Ishiguro K, Sartorelli A C. J Med Chem. 1985;28:1194–1198. doi: 10.1021/jm00147a012. [DOI] [PubMed] [Google Scholar]
- 36.Hughes M N, Nicklin H G. J. Chem. Soc. 1968. 450–452. [Google Scholar]
- 37.Ikehara M, Tada H, Muneyama K. Chem Pharm Bull. 1965;13:1140–1142. doi: 10.1248/cpb.13.1140. [DOI] [PubMed] [Google Scholar]
- 38.Levene P A, Tipson R S. J Biol Chem. 1931;92:109–115. [Google Scholar]
- 39.Raoul S, Berger M, Buchko G W, Joshi P C, Morin B, Weinfeld M, Cadet J. J. Chem. Soc. Perkin Trans. 2. 1996. 371–381. [Google Scholar]
- 40.Tretyakova N Y, Niles J C, Burney S, Wishnok J S, Tannenbaum S R. Chem Res Toxicol. 1999;12:459–466. doi: 10.1021/tx980235c. [DOI] [PubMed] [Google Scholar]
- 41.Evans J, Maccabee M, Hatahet Z, Courcelle J, Bockrath R, Ide H, Wallace S. Mutat Res. 1993;299:147–156. doi: 10.1016/0165-1218(93)90092-r. [DOI] [PubMed] [Google Scholar]