Abstract
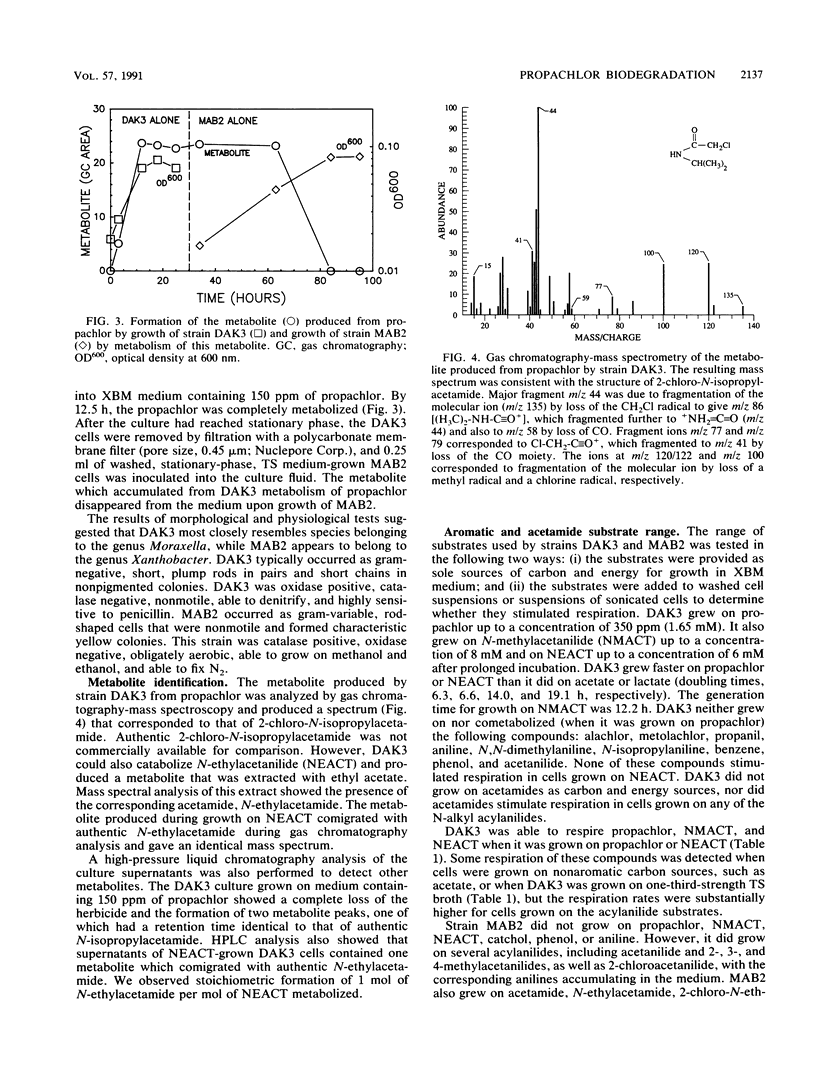
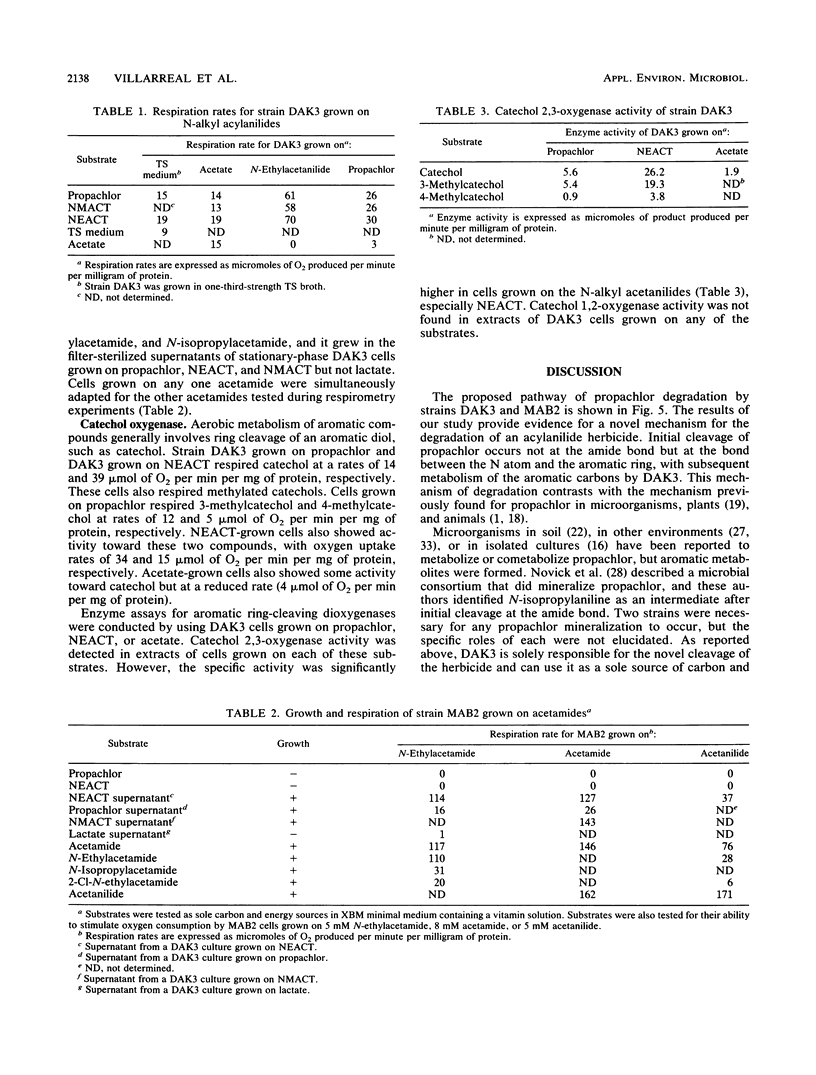
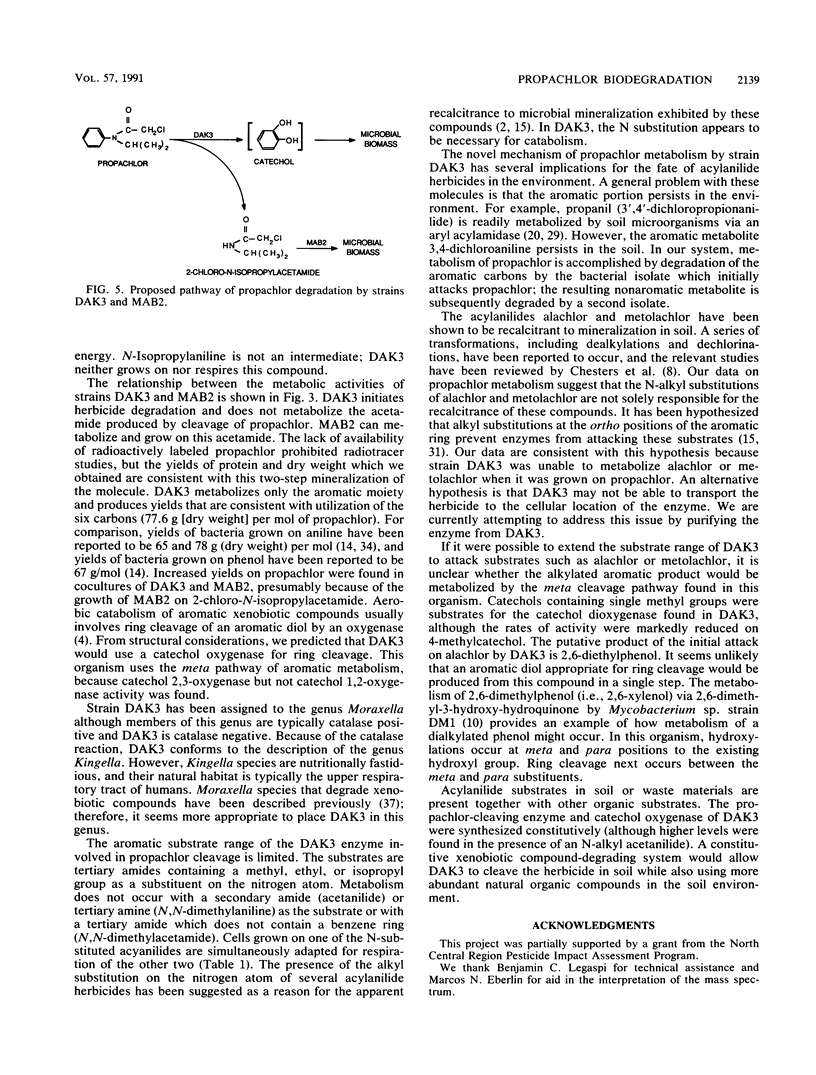
Soil from a pesticide disposal site was used to enrich for microorganisms that degraded the acylanilide herbicide propachlor (2-chloro-N-isopropylacetanilide). After seven transfers of the enrichment, the culture contained about six strains. The highest yield of microbial biomass occurred if just two of these isolates, strains DAK3 and MAB2, were inoculated into a mineral salts medium containing propachlor. When only strain DAK3 was grown on propachlor, a metabolite (2-chloro-N-isopropylacetamide) was released into the medium. Strain MAB2 could grow on this metabolite. The results of morphological and physiological tests suggest that strains DAK3 and MAB2 most closely resemble species belonging to the genera Moraxella and Xanthobacter, respectively. Strain DAK3 can respire and grow on N-substituted acylanilides containing methyl, ethyl, or isopropyl substitutions, but is incapable of respiration or growth on acetanilide, aniline, or the acylanilide herbicides alachlor and metolachlor. Strain DAK3 appears to use the aromatic C atoms of propachlor for growth, as suggested by the growth yield on propachlor and the induction of catechol 2,3-oxygenase activity in acylanilide-grown cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakke J. E., Gustafsson J. A., Gustafsson B. E. Metabolism of propachlor by the germfree rat. Science. 1980 Oct;210(4468):433–435. doi: 10.1126/science.7433983. [DOI] [PubMed] [Google Scholar]
- Bartha R. Fate of herbicide-derived chloroanilines in soil. J Agric Food Chem. 1971 Mar-Apr;19(2):385–387. doi: 10.1021/jf60174a024. [DOI] [PubMed] [Google Scholar]
- Chesters G., Simsiman G. V., Levy J., Alhajjar B. J., Fathulla R. N., Harkin J. M. Environmental fate of alachlor and metolachlor. Rev Environ Contam Toxicol. 1989;110:1–74. doi: 10.1007/978-1-4684-7092-5_1. [DOI] [PubMed] [Google Scholar]
- Engelhardt G., Wallnöfer P. R., Plapp R. Purification and properties of an aryl acylamidase of Bacillus sphaericus, catalyzing the hydrolysis of various phenylamide herbicides and fungicides. Appl Microbiol. 1973 Nov;26(5):709–718. doi: 10.1128/am.26.5.709-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers J., Rubio M. A., Knackmuss H. J., Freier-Schröder D. Bacterial metabolism of 2,6-xylenol. Appl Environ Microbiol. 1989 Nov;55(11):2904–2908. doi: 10.1128/aem.55.11.2904-2908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAISHI O., KATAGIRI M., ROTHBERG S. Studies on oxygenases; pyrocatechase. J Biol Chem. 1957 Dec;229(2):905–920. [PubMed] [Google Scholar]
- Hammond P. M., Price C. P., Scawen M. D. Purification and properties of aryl acylamidase from Pseudomonas fluorescens ATCC 39004. Eur J Biochem. 1983 May 16;132(3):651–655. doi: 10.1111/j.1432-1033.1983.tb07413.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzilotta R. P., Pramer D. Herbicide transformation. I. Studies with whole cells of Fusarium solani. Appl Microbiol. 1970 Feb;19(2):301–306. doi: 10.1128/am.19.2.301-306.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzilotta R. P., Pramer D. Herbicide transformation. II. Studies with an acylamidase of Fusarium solani. Appl Microbiol. 1970 Feb;19(2):307–313. doi: 10.1128/am.19.2.307-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Zheng Z., Zhang R., Bollag J. M. Sorption and metabolism of metolachlor by a bacterial community. Appl Environ Microbiol. 1989 Mar;55(3):733–740. doi: 10.1128/aem.55.3.733-740.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick N. J., Alexander M. Cometabolism of low concentrations of propachlor, alachlor, and cycloate in sewage and lake water. Appl Environ Microbiol. 1985 Apr;49(4):737–743. doi: 10.1128/aem.49.4.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Saxena A., Zhang R. W., Bollag J. M. Microorganisms capable of metabolizing the herbicide metolachlor. Appl Environ Microbiol. 1987 Feb;53(2):390–396. doi: 10.1128/aem.53.2.390-396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen W. C., Collette T. W. Microbial degradation of seven amides by suspended bacterial populations. Appl Environ Microbiol. 1989 Oct;55(10):2545–2549. doi: 10.1128/aem.55.10.2545-2549.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


