Abstract
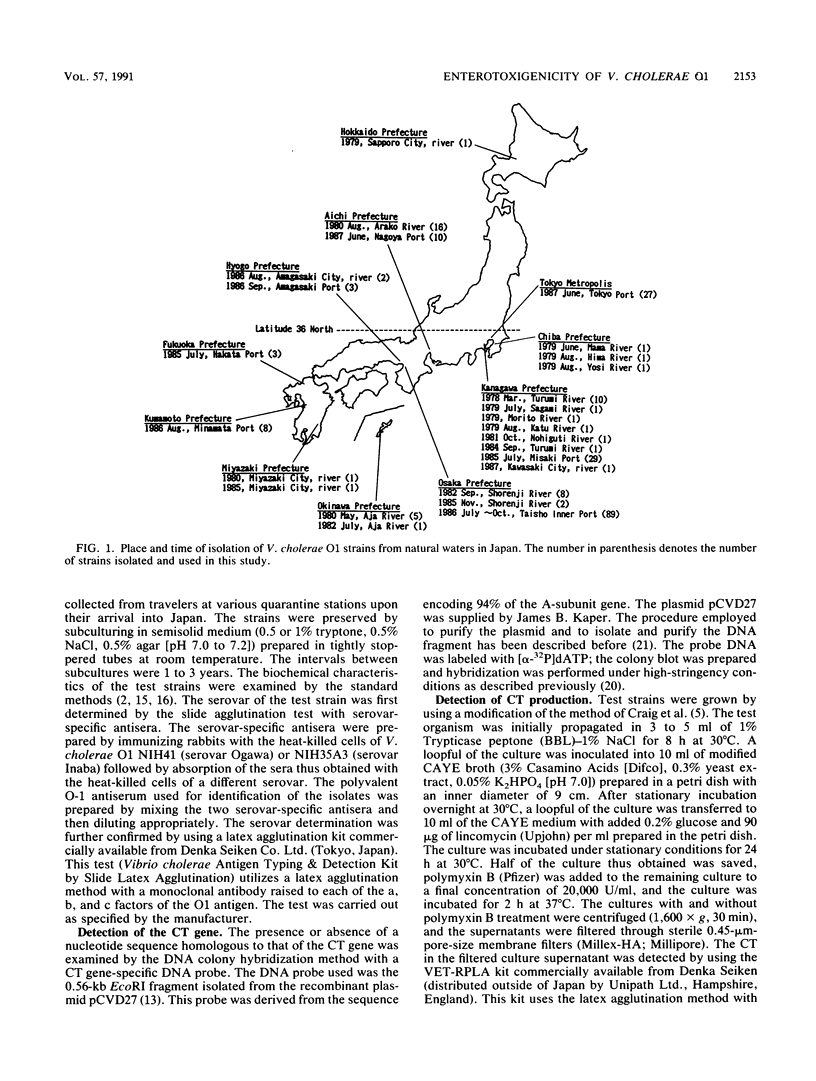
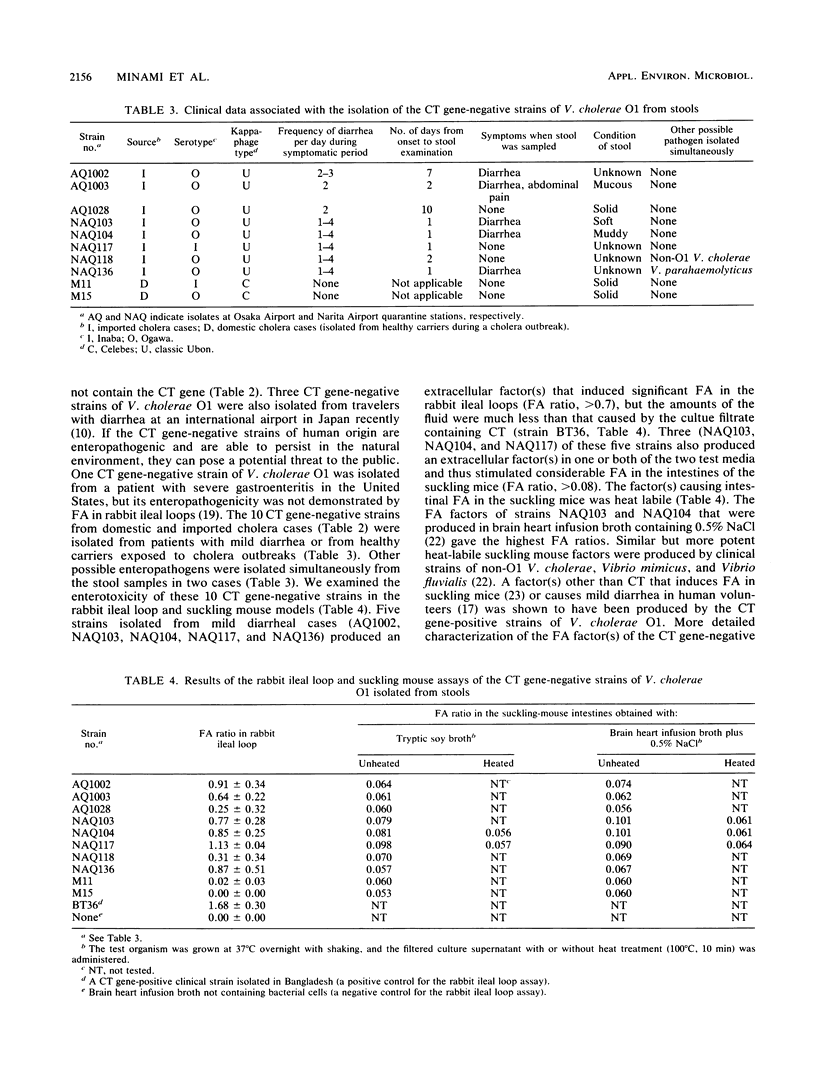
Vibrio cholerae O1 strains isolated from various sources in Japan over the years 1977 through 1987 were examined to confirm the presence or absence of the cholera enterotoxin (CT) gene and production of CT and to determine the kappa-phage type. The CT gene was detected in none of 225 isolates from natural waters but was present in all of the 10 isolates from environmental waters implicated in domestic cholera cases, in 64 strains (26.6%) of the 241 isolates from imported seafoods, in 43 strains (95.6%) of the 45 isolates from domestic cholera cases, and in 119 strains (93.7%) of the 127 isolates from imported cholera cases. The results suggest that the CT gene-positive strains of V. cholerae O1 have been imported into Japan through seafoods and/or by travelers. Sporadic cholera cases have resulted in contamination of the surrounding environment, but the CT gene-positive strains may not have persisted in natural waters to serve as a reservoir for epidemic cholera. The commercially available VET-RPLA kit (a latex agglutination kit for immunological detection of CT) detected production of CT in all of the CT gene-positive strains, indicating that there was no silent CT gene in the test strains. There was a strong correlation between the kappa-phage type and the presence or absence of the CT gene, suggesting a significant clonal difference between CT gene-positive and -negative strains. Five CT gene-negative strains isolated from imported cholera cases (travelers with mild diarrhea) induced a considerable amount of fluid accumulation in rabbit and/or suckling mouse intestines, indicating production of an enterotoxic factor(s) other than CT.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. J., Hickman-Brenner F. W., Sowers E. G., Puhr N. D., Farmer J. J., 3rd, Wachsmuth I. K. Comparison of a latex agglutination assay and an enzyme-linked immunosorbent assay for detecting cholera toxin. J Clin Microbiol. 1990 Jan;28(1):128–130. doi: 10.1128/jcm.28.1.128-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAM W. E., Jr Effect of excess nitrite on tests for indole and the cholera red reaction. J Bacteriol. 1959 Mar;77(3):328–330. doi: 10.1128/jb.77.3.328-330.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Wachsmuth K., Davis B. R., Bopp C. A., Chaiken B. P., Lee J. V. Toxigenic Vibrio cholerae O1 strain from Mexico identical to United States isolates. Lancet. 1983 Oct 15;2(8355):912–912. doi: 10.1016/s0140-6736(83)90894-2. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Yamamoto K., Takeda Y., Miwatani T. Production of cholera-like enterotoxin by a Vibrio cholerae non-O1 strain isolated from the environment. Infect Immun. 1981 Oct;34(1):90–97. doi: 10.1128/iai.34.1.90-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Desmarchelier P. M., Senn C. R. A molecular epidemiological study of Vibrio cholerae in Australia. Med J Aust. 1989 Jun 5;150(11):631–634. doi: 10.5694/j.1326-5377.1989.tb136726.x. [DOI] [PubMed] [Google Scholar]
- Honda S., Shimoirisa K., Adachi A., Saito K., Asano N., Taniguchi T., Honda T., Miwatani T. Clinical isolates of Vibrio cholerae O1 not producing cholera toxin. Lancet. 1988 Dec 24;2(8626-8627):1486–1486. doi: 10.1016/s0140-6736(88)90955-5. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Powers C., Bryant R. G., Abbott S. L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988 Jul;1(3):245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Moseley S. L., Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981 May;32(2):661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. Y., Morris J. G., Jr, Kaper J. B., Gross T., Michalski J., Morrison C., Libonati J. P., Israel E. Persistence of cholera in the United States: isolation of Vibrio cholerae O1 from a patient with diarrhea in Maryland. J Clin Microbiol. 1986 Mar;23(3):624–626. doi: 10.1128/jcm.23.3.624-626.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Picardi J. L., Lieb S., Lee J. V., Roberts A., Hood M., Gunn R. A., Blake P. A. Isolation of nontoxigenic Vibrio cholerae O group 1 from a patient with severe gastrointestinal disease. J Clin Microbiol. 1984 Feb;19(2):296–297. doi: 10.1128/jcm.19.2.296-297.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Ishibashi M., Takeda Y., Kaper J. B. Detection of the thermostable direct hemolysin gene and related DNA sequences in Vibrio parahaemolyticus and other vibrio species by the DNA colony hybridization test. Infect Immun. 1985 Sep;49(3):481–486. doi: 10.1128/iai.49.3.481-486.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Kaper J. B. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):558–564. doi: 10.1128/jb.162.2.558-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J. Medium-dependent production of extracellular enterotoxins by non-O-1 Vibrio cholerae, Vibrio mimicus, and Vibrio fluvialis. Appl Environ Microbiol. 1983 Jan;45(1):228–231. doi: 10.1128/aem.45.1.228-231.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J., Rollins D. M., Joseph S. W. Vibrio factors cause rapid fluid accumulation in suckling mice. Infect Immun. 1983 Jun;40(3):1083–1091. doi: 10.1128/iai.40.3.1083-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., SHIMODORI S. "PROPHAGE-TYPING" OF EL TOR VIBRIOS. J Bacteriol. 1963 Apr;85:957–958. doi: 10.1128/jb.85.4.957-958.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam W. C., Lung M. L., Ng K. Y., Ng M. H. Molecular epidemiology of Vibrio cholerae in Hong Kong. J Clin Microbiol. 1989 Aug;27(8):1900–1902. doi: 10.1128/jcm.27.8.1900-1902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


