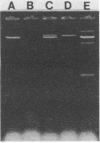
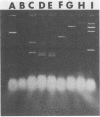
Abstract
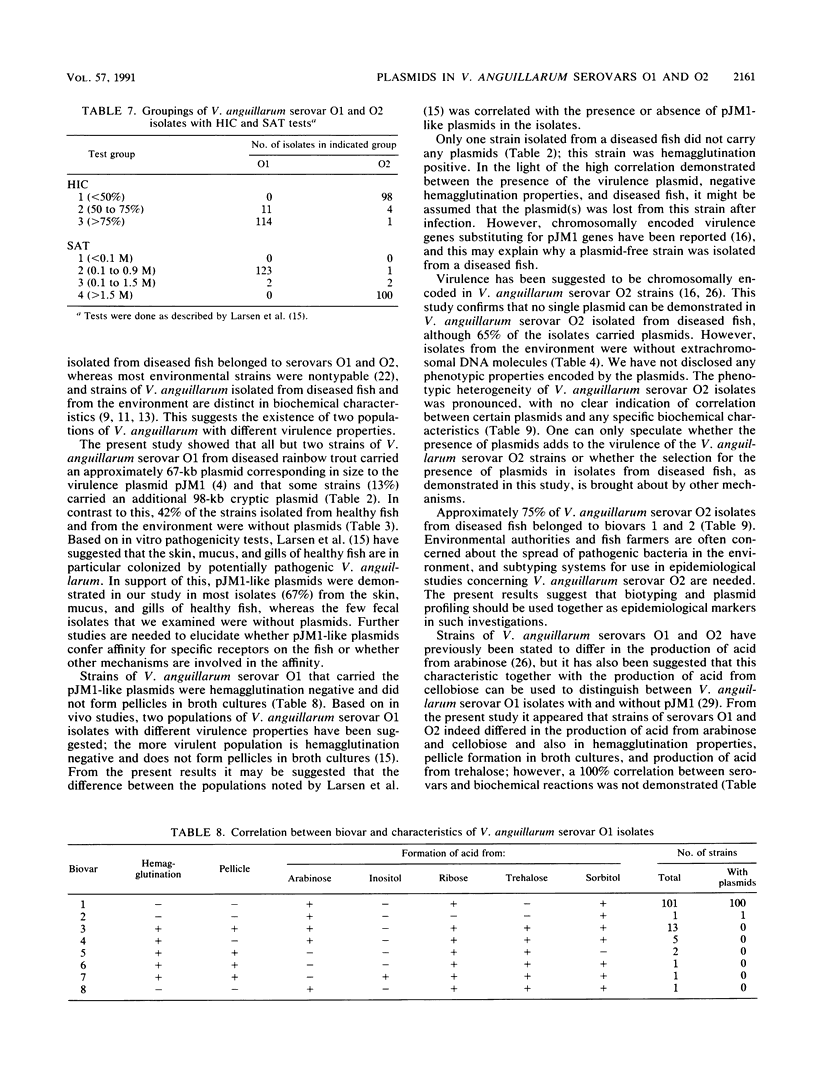
Two hundred and twenty-eight isolates of Vibrio anguillarum serovar O1 (125 isolates) and serovar O2 (103 isolates) have been characterized with regard to plasmid contents, biochemical properties, and in vitro hemagglutination and hydrophobic properties. Among 74 V. anguillarum isolates from diseased fish, 63 carried only a 67-kb plasmid (pJM1), 9 carried an additional 98-kb plasmid, and 1 isolate carried only the 98-kb plasmid. Only one isolate was without plasmids. In V. anguillarum serovar O1 from nondiseased fish (mucus and gills), plasmids of the same sizes were present in 29 isolates (58%), whereas 21 isolates (42%) were plasmid free. Based on hemagglutination and biochemical properties, V. anguillarum serovar O1 isolates were divided into eight biovars. The plasmid-carrying strains (102 isolates) all fell within biovars 1 and 2, whereas the 23 strains of biovars 3 to 8 were without plasmids. It was tentatively concluded there are two populations of V. anguillarum serovar O1. One population contains plasmid(s), is hemagglutination negative and trehalose negative, and does not form pellicles in broth cultures, whereas the other population is plasmid free and has the opposite characteristics. The former group is the one related to disease in fish. All 20 V. anguillarum serovar O2 isolates from the environment were without plasmids, whereas 54 (65%) of the isolates from fish (trout and cod) carried plasmids. The biochemical diversity within serovar O2 was pronounced; 13 different biovars were demonstrated. No correlation between the presence of plasmids and biochemical properties was observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Hodges L. L., Schiewe M. H. Curing of a plasmid is correlated with an attenuation of virulence in the marine fish pathogen Vibrio anguillarum. Infect Immun. 1980 Mar;27(3):897–902. doi: 10.1128/iai.27.3.897-902.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Schiewe M. H., Falkow S. Evidence for plasmid contribution to the virulence of fish pathogen Vibrio anguillarum. Infect Immun. 1977 Nov;18(2):509–513. doi: 10.1128/iai.18.2.509-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. L., Jensen N. J. The ulcus-syndrome in cod (Gadus morhua). II. A bacteriological investigation. Nord Vet Med. 1979 Jul-Aug;31(7-8):289–296. [PubMed] [Google Scholar]
- Larsen J. L., Jensen N. J. The ulcus-syndrome in cod (Gadus morhua). V. Prevalence in selected Danish marine recipients and a control site in the period 1976--1979. Nord Vet Med. 1982 Jul-Sep;34(7-9):303–312. [PubMed] [Google Scholar]
- Larsen J. L., Rasmussen H. B., Dalsgaard I. Study of Vibrio anguillarum strains from different sources with emphasis on ecological and pathobiological properties. Appl Environ Microbiol. 1988 Sep;54(9):2264–2267. doi: 10.1128/aem.54.9.2264-2267.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos M. L., Salinas P., Toranzo A. E., Barja J. L., Crosa J. H. Chromosome-mediated iron uptake system in pathogenic strains of Vibrio anguillarum. J Bacteriol. 1988 Apr;170(4):1920–1925. doi: 10.1128/jb.170.4.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. E., Larsen J. L. Restriction fragment length polymorphism of the Vibrio anguillarum serovar O1 virulence plasmid. Appl Environ Microbiol. 1990 Oct;56(10):3130–3132. doi: 10.1128/aem.56.10.3130-3132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle P. A., Fry J. C., Day M. J., Bale M. J. An accurate method for estimating sizes of small and large plasmids and DNA fragments by gel electrophoresis. J Gen Microbiol. 1986 Jan;132(1):53–59. doi: 10.1099/00221287-132-1-53. [DOI] [PubMed] [Google Scholar]
- Sørensen U. B., Larsen J. L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986 Mar;51(3):593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Rowe B., Ferguson J. L., Ward L. R. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J Hyg (Lond) 1986 Dec;97(3):419–426. doi: 10.1017/s0022172400063609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Potter S. A., Colwell R. R., Hetrick F. M., Crosa J. H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983 Mar;39(3):1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Courtice I. D., Khouri A. G., Crosa J. H., Schiewe M. H. Serum resistance and hemagglutination ability of marine vibrios pathogenic for fish. Infect Immun. 1981 Dec;34(3):702–707. doi: 10.1128/iai.34.3.702-707.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. A., Potter S. A., Crosa J. H. Iron uptake system medicated by Vibrio anguillarum plasmid pJM1. J Bacteriol. 1983 Nov;156(2):880–887. doi: 10.1128/jb.156.2.880-887.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiik R., Hoff K. A., Andersen K., Daae F. L. Relationships between plasmids and phenotypes of presumptive strains of Vibrio anguillarum isolated from different fish species. Appl Environ Microbiol. 1989 Apr;55(4):826–831. doi: 10.1128/aem.55.4.826-831.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Crosa J. H. Evidence for the role of a siderophore in promoting Vibrio anguillarum infections. J Gen Microbiol. 1986 Oct;132(10):2949–2952. doi: 10.1099/00221287-132-10-2949. [DOI] [PubMed] [Google Scholar]