Abstract
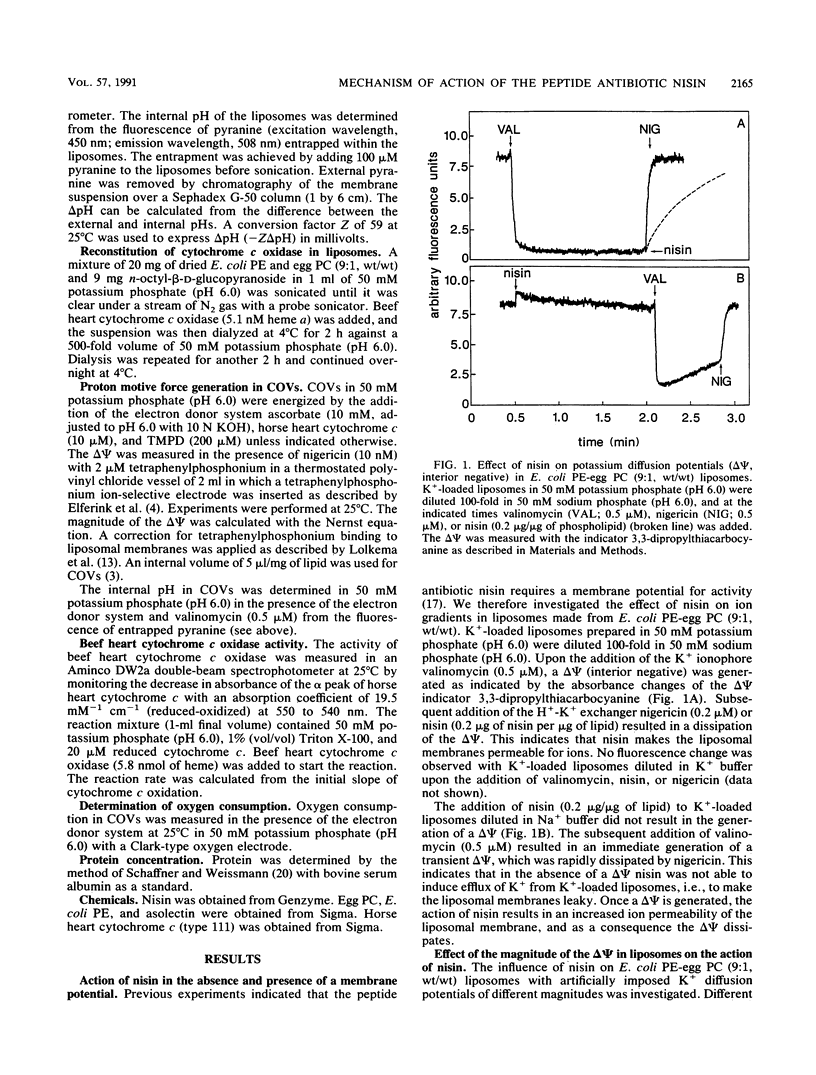
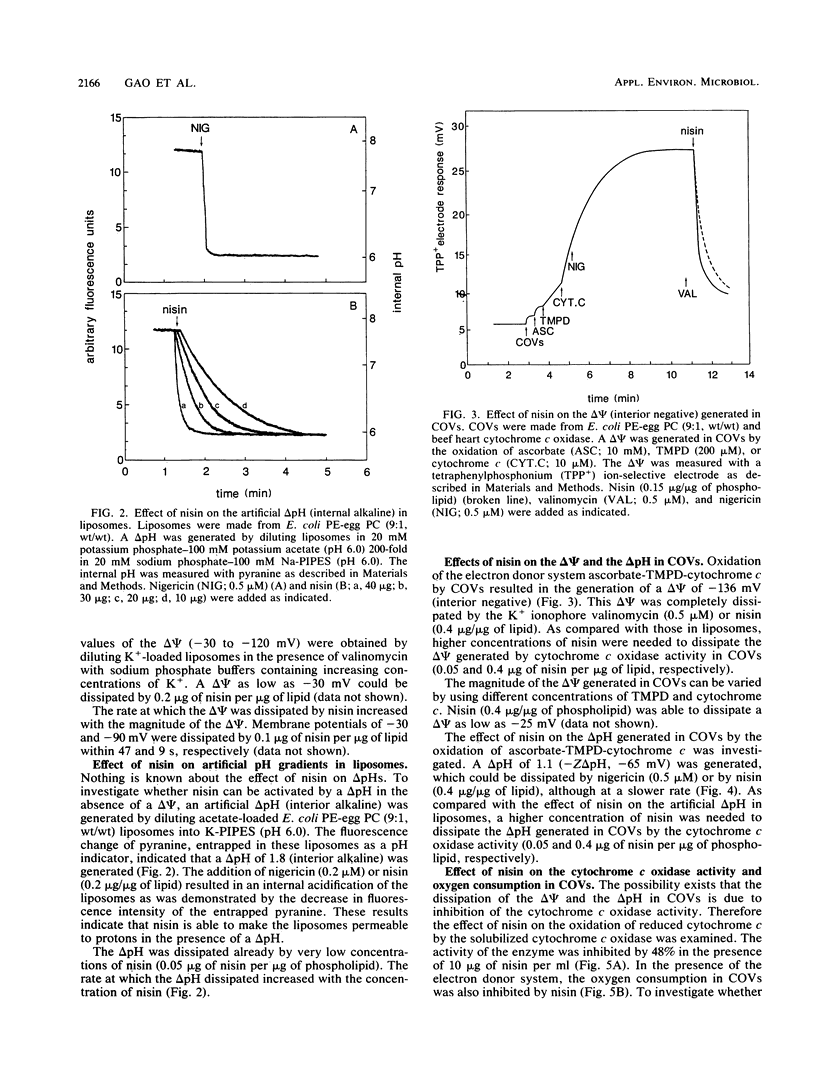
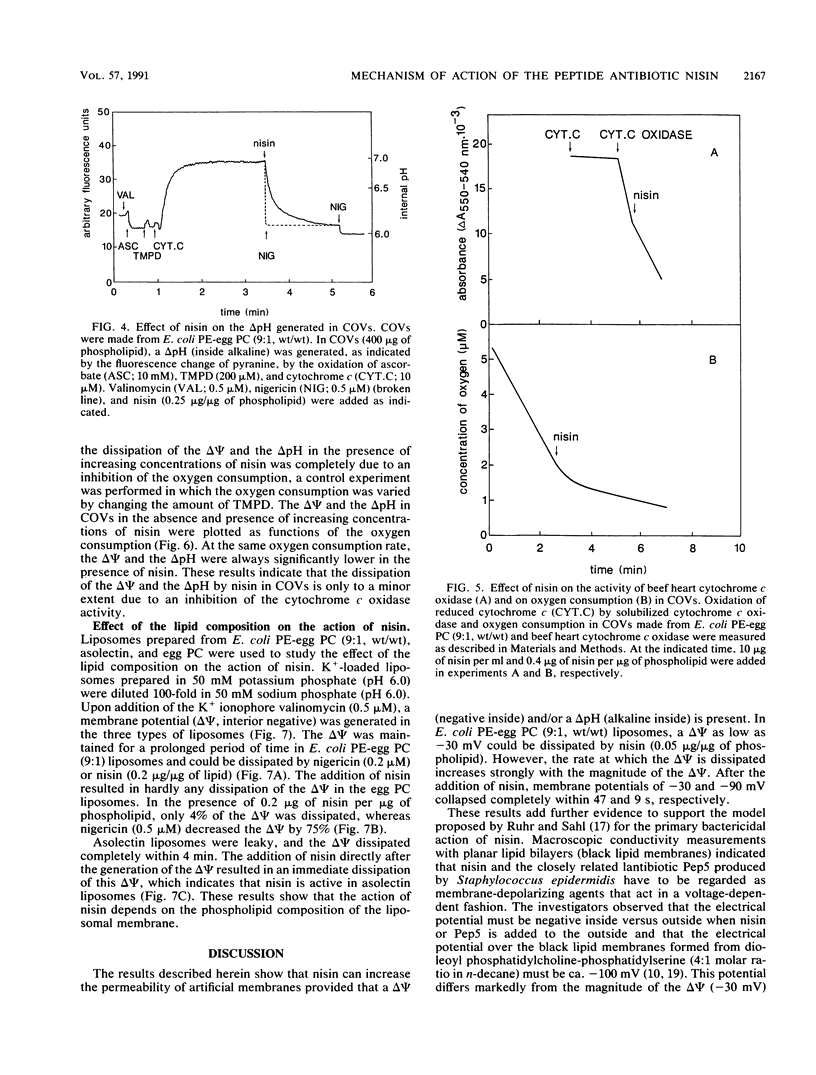
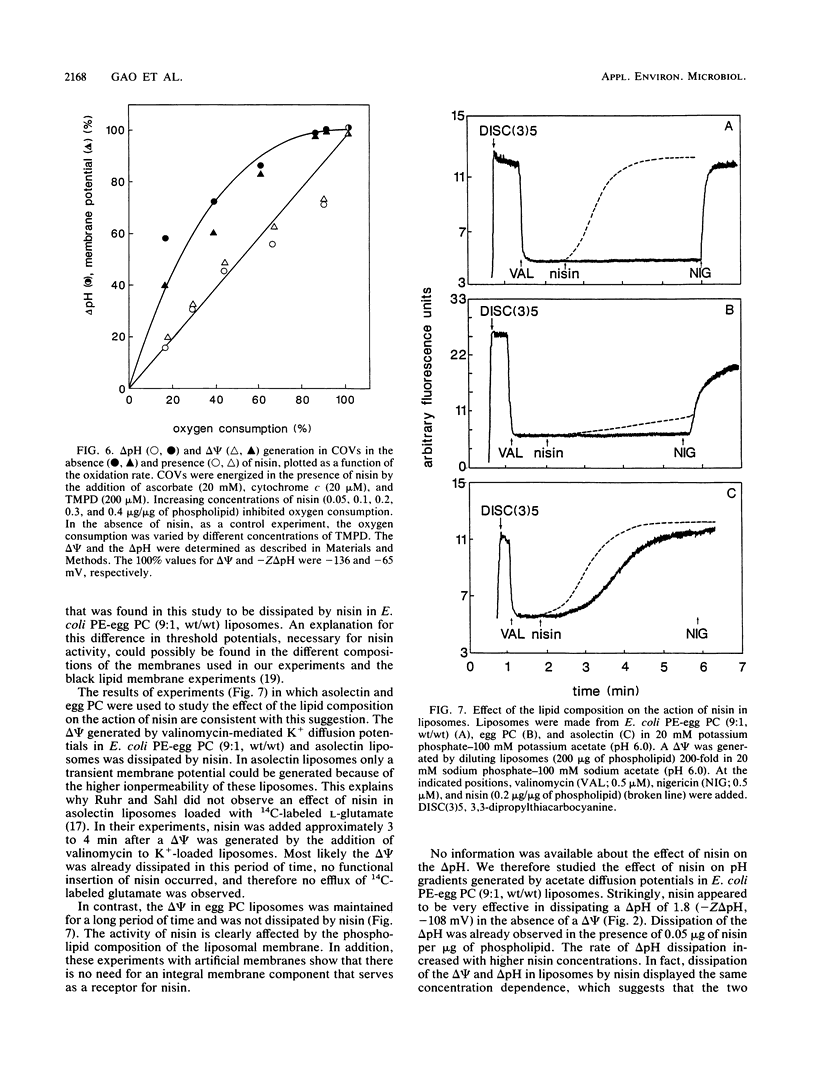
The interaction of the peptide antibiotic nisin with liposomes has been studied. The effect of this interaction was analyzed on the membrane potential (inside negative) and the pH gradient (inside alkaline) in liposomes made from Escherichia coli phosphatidylethanolamine and egg phosphatidylcholine (9:1, wt/wt). The membrane potential and pH gradient were generated by artificial ion gradients or by the oxidation of ascorbate, N,N,N',N'-tetramethyl-p-phenylenediamine, and cytochrome c by the beef heart cytochrome c oxidase incorporated in the liposomal membranes. Nisin dissipated the membrane potential and the pH gradient in both types of liposomes and inhibited oxygen consumption by cytochrome c oxidase in proteoliposomes. The dissipation of the proton motive force in proteoliposomes was only to a minor extent due to a decrease of the oxidase activity by nisin. The results in these model systems show that a membrane potential and/or a pH gradient across the membrane enhances the activity of nisin. Nisin incorporates into the membrane and makes the membrane permeable for ions. As a result, both the membrane potential and pH gradient are dissipated. The activity of nisin was found to be influenced by the phospholipid composition of the liposomal membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRIDGE N. J., NEWTON G. G. F., ABRAHAM E. P. Purification and nature of the antibiotic nisin. Biochem J. 1952 Dec;52(4):529–535. doi: 10.1042/bj0520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein R. O., Koehler T. M., Collier R. J., Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl Environ Microbiol. 1990 Aug;56(8):2551–2558. doi: 10.1128/aem.56.8.2551-2558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMSEIER H. R. [The effect of nisin on Clostridium butyricum Prazm]. Arch Mikrobiol. 1960;37:57–94. doi: 10.1007/BF00414627. [DOI] [PubMed] [Google Scholar]
- Reisinger P., Seidel H., Tschesche H., Hammes W. P. The effect of nisin on murein synthesis. Arch Microbiol. 1980 Oct;127(3):187–193. doi: 10.1007/BF00427192. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Bactericidal cationic peptides involved in bacterial antagonism and host defence. Microbiol Sci. 1985 Jul;2(7):212–217. [PubMed] [Google Scholar]
- Sahl H. G., Kordel M., Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149(2):120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



