Abstract
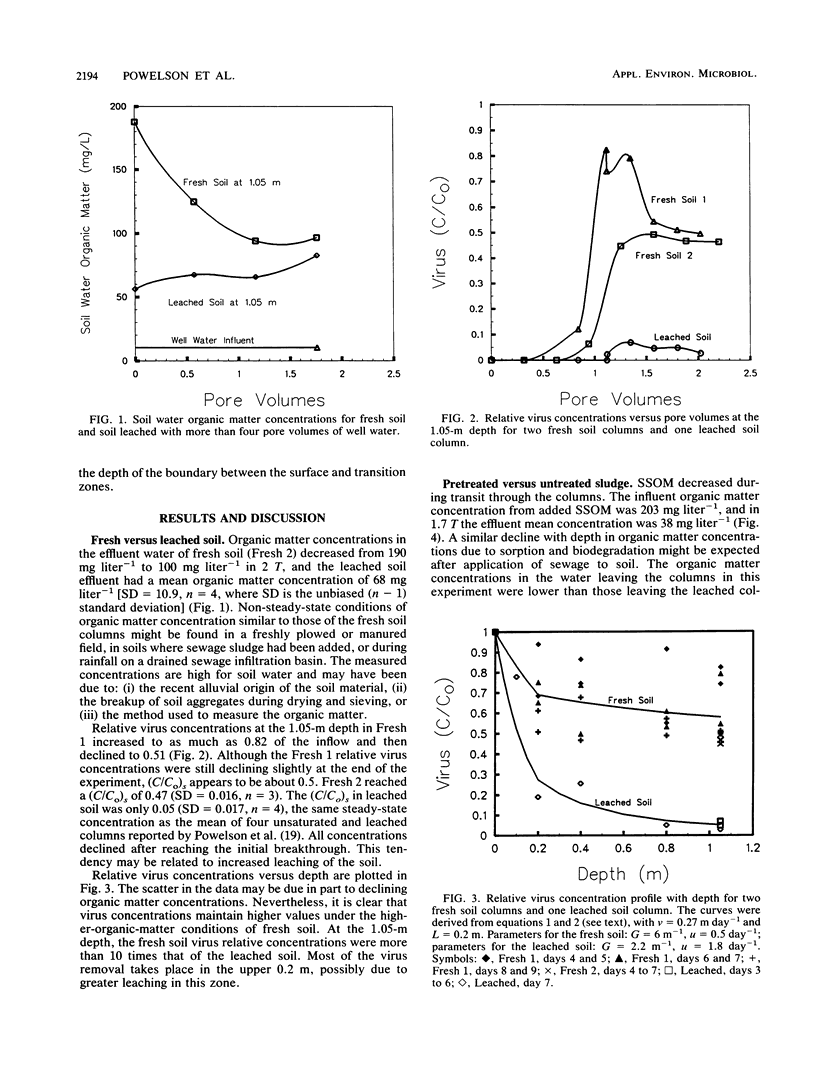
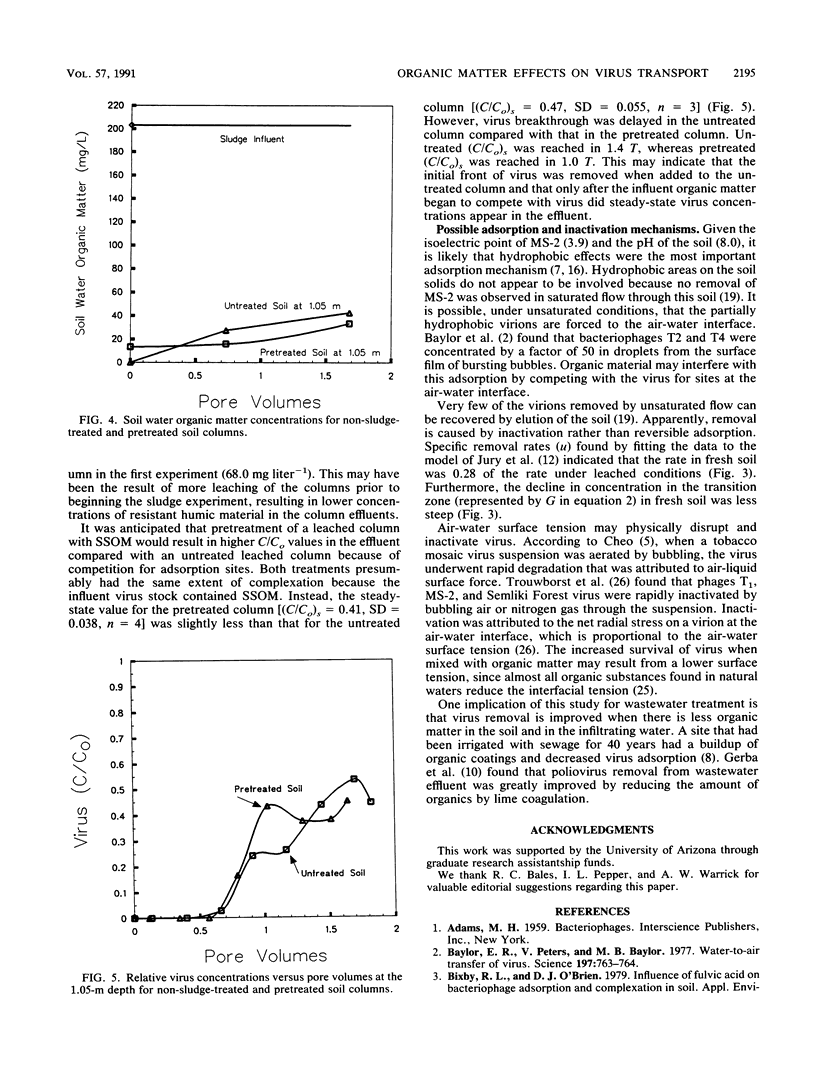
The effects of natural humic material and sewage sludge organic matter (SSOM) derived from primary treated sewage sludge on virus transport by unsaturated flow through soil columns were evaluated. Bacteriophage MS-2 was applied to loamy fine sand columns 0.052 m in diameter and 1.05 m long. Virus concentrations in the influent and effluent were measured daily for 7 to 9 days. In the first experiment, virus transport through two fresh soil columns was compared with that through a column previously leached with more than four pore volumes (T) of well water. The soil water organic matter concentrations in the leachate of the fresh soil declined with time. Relative virus concentrations (C/Co) from one fresh soil column reached 0.82 in 0.9 T and then declined to 0.51 by 2.1 T. The other fresh soil column reached and maintained a steady-state relative virus concentration [(C/Co)s] of 0.47 from 1.5 to 2.5 T. The leached column reached and maintained a (C/Co)s of 0.05. Concentrations measured at 0.2-, 0.4-, 0.8-, and 1.05-m depths indicated that most virus particles were removed in the surface 0.2 m. In the second experiment, one leached column was pretreated with SSOM derived from primary treated sewage sludge and the other leached column was untreated. SSOM concentrations declined with depth. A suspension of virus and SSOM in well water was applied to both columns. Although the (C/Co)s values were similar (0.41 for the pretreated column and 0.47 for the untreated column), breakthrough was delayed for the untreated column. Both natural humic material and sewage sludge-derived SSOM increased the unsaturated-flow transport of MS-2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor E. R., Peters V., Baylor M. B. Water-to-air transfer of virus. Science. 1977 Aug 19;197(4305):763–764. doi: 10.1126/science.329413. [DOI] [PubMed] [Google Scholar]
- Duboise S. M., Moore B. E., Sagik B. P. Poliovirus survival and movement in a sandy forest soil. Appl Environ Microbiol. 1976 Apr;31(4):536–543. doi: 10.1128/aem.31.4.536-543.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Shah D. O., Ingram L. O. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Bass N., Catalano-Sherman J. Humic acid interference with virus recovery by electropositive microporous filters. Appl Environ Microbiol. 1986 Sep;52(3):556–561. doi: 10.1128/aem.52.3.556-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J. C., Gerba C. P. Effect of ionic composition of suspending solution on virus adsorption by a soil column. Appl Environ Microbiol. 1984 Mar;47(3):484–488. doi: 10.1128/aem.47.3.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Thomas M. Z., Beckwith C. A. Adsorption of enteroviruses to soil cores and their subsequent elution by artificial rainwater. Appl Environ Microbiol. 1979 Oct;38(4):680–687. doi: 10.1128/aem.38.4.680-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. I. Properties of the viral particles. J Bacteriol. 1966 Jan;91(1):442–448. doi: 10.1128/jb.91.1.442-448.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Hickey A. R. Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Appl Environ Microbiol. 1985 Feb;49(2):259–264. doi: 10.1128/aem.49.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. H., Wallis C., Ward C. H. Inactivation of clay-associated bacteriophage MS-2 by chlorine. Appl Environ Microbiol. 1977 Feb;33(2):385–391. doi: 10.1128/aem.33.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouwborst T., Kuyper S., de Jong J. C., Plantinga A. D. Inactivation of some bacterial and animal viruses by exposure to liquid-air interfaces. J Gen Virol. 1974 Jul;24(1):155–165. doi: 10.1099/0022-1317-24-1-155. [DOI] [PubMed] [Google Scholar]
- Yates M. V., Gerba C. P., Kelley L. M. Virus persistence in groundwater. Appl Environ Microbiol. 1985 Apr;49(4):778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



