Abstract
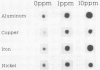
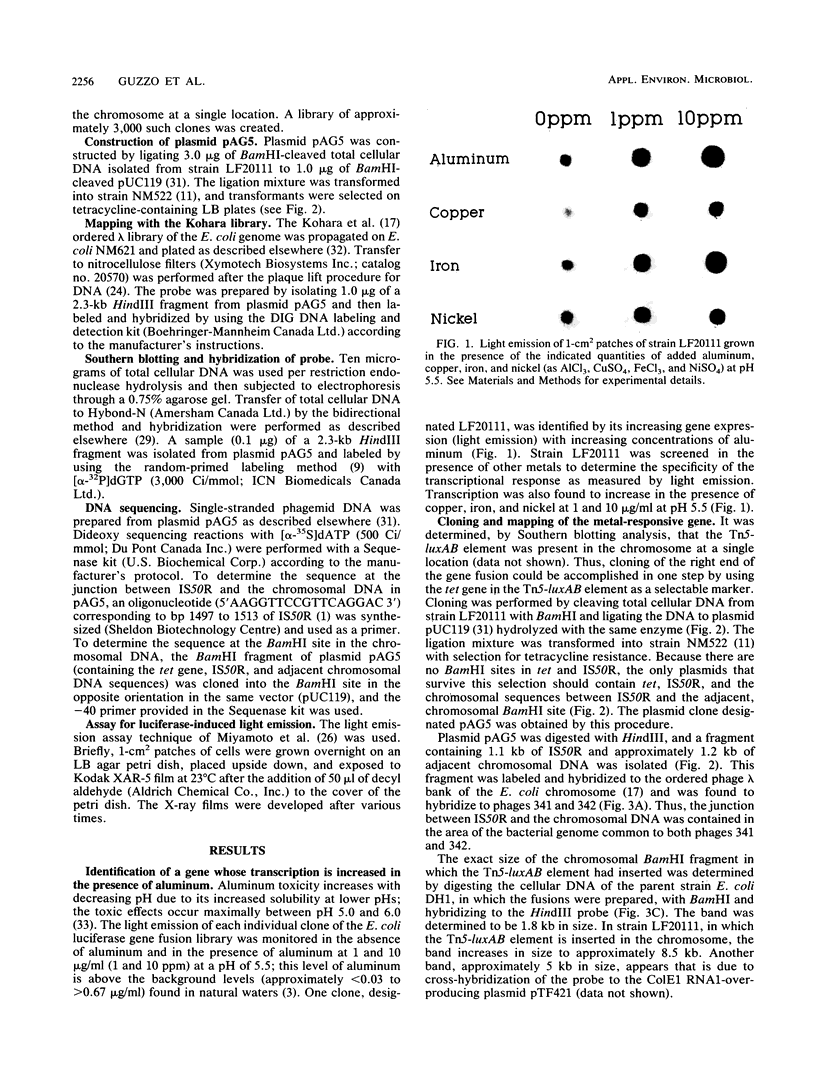
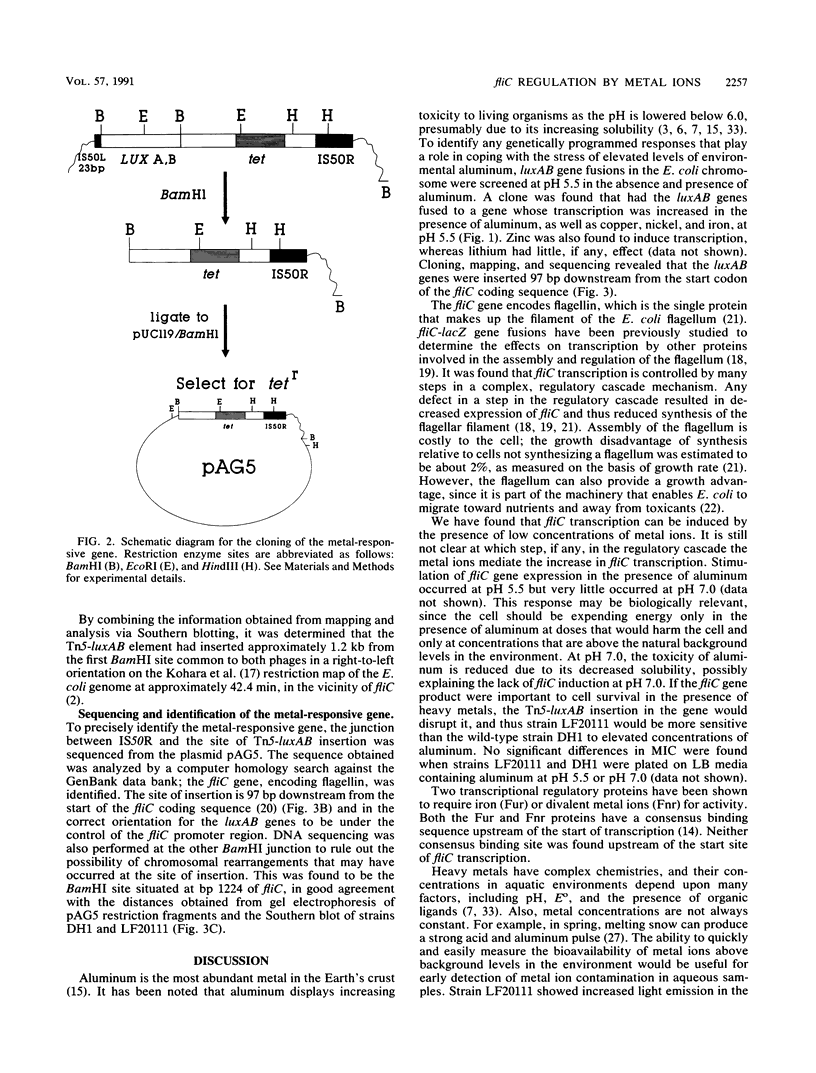
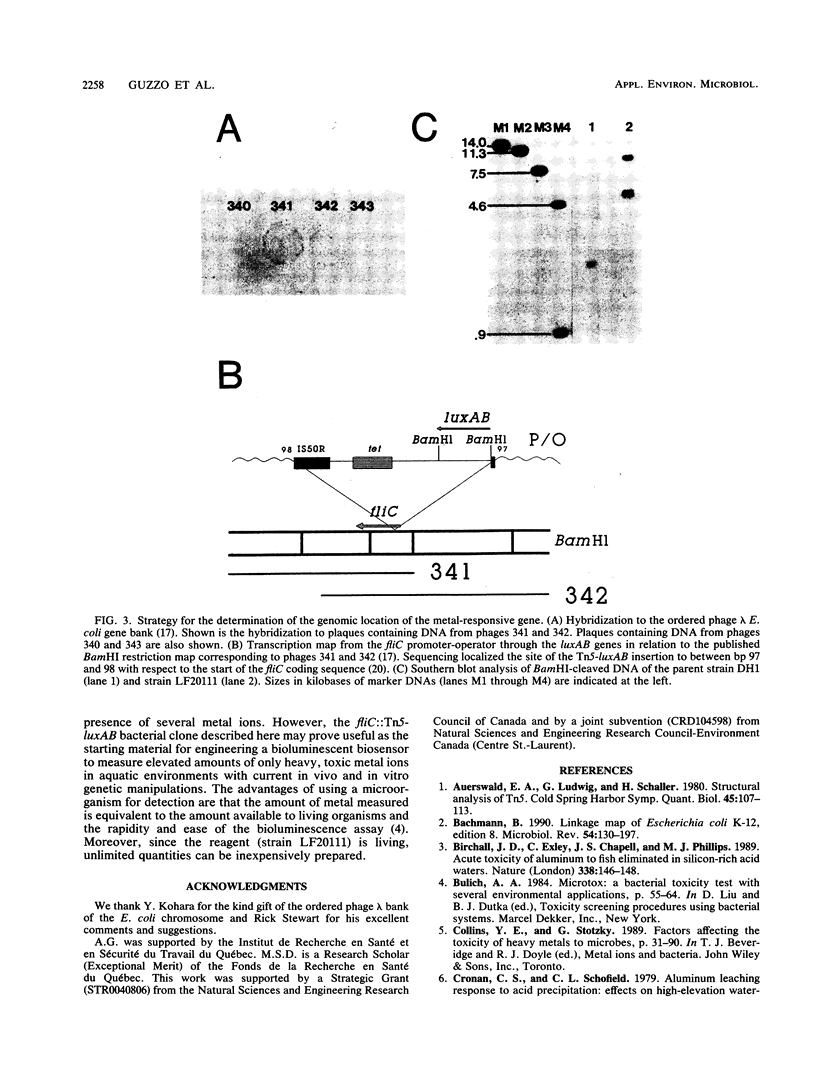
luxAB gene fusions in the Escherichia coli genome were used to screen for clones displaying transcriptional changes in the presence of aluminum. One clone was found that contained a luciferase gene fusion in which transcription was increased in the presence of aluminum and which was subsequently shown to be induced by copper, iron, and nickel. Cloning of the metal-regulated gene, hybridization to the ordered phage lambda bank of the E. coli chromosome, and sequencing of DNA adjacent to the luxAB fusion revealed that the insertion occurred within the fliC (hag) gene of E. coli. This gene encodes flagellin, the filament subunit of the bacterial motility organ, and is under the control of several regulatory cascades. These results suggest that environmental metals may play a role in the regulation of the motility potential of E. coli and that this bioluminescent gene fusion clone (or derivatives thereof) may be used to prepare a biosensor for the rapid detection of metal contamination in water samples.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fitzwater T., Tamm J., Polisky B. RNA1 is sufficient to mediate plasmid ColE1 incompatibility in vivo. J Mol Biol. 1984 May 25;175(3):409–417. doi: 10.1016/0022-2836(84)90357-7. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hennecke H. Regulation of bacterial gene expression by metal-protein complexes. Mol Microbiol. 1990 Oct;4(10):1621–1628. doi: 10.1111/j.1365-2958.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. C., Wood M. DNA, a Possible Site of Action of Aluminum in Rhizobium spp. Appl Environ Microbiol. 1990 Dec;56(12):3629–3633. doi: 10.1128/aem.56.12.3629-3633.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Reznikoff W. S. DNA sequences at the ends of transposon Tn5 required for transposition. Nature. 1983 Jul 21;304(5923):280–282. doi: 10.1038/304280a0. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Komeda Y. Fusions of flagellar operons to lactose genes on a mu lac bacteriophage. J Bacteriol. 1982 Apr;150(1):16–26. doi: 10.1128/jb.150.1.16-26.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G., Asaka J., Fujiwara T., Fujiwara T., Node K., Kondo E. Nucleotide sequence of the hag gene encoding flagellin of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1479–1483. doi: 10.1128/jb.168.3.1479-1483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. D., Boylan M., Evans J. F., Hasel K. W., Meighen E. A., Graham A. F. Polycistronic mRNAs code for polypeptides of the Vibrio harveyi luminescence system. J Bacteriol. 1985 Mar;161(3):995–1001. doi: 10.1128/jb.161.3.995-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D. W. Effects of Acid rain on freshwater ecosystems. Science. 1988 Jan 8;239(4836):149–157. doi: 10.1126/science.239.4836.149. [DOI] [PubMed] [Google Scholar]
- Silver S., Misra T. K. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Tolias P. P., Dubow M. S. The amino terminus of the bacteriophage D108 transposase protein contains a two-component sequence-specific DNA-binding domain. Virology. 1987 Mar;157(1):117–126. doi: 10.1016/0042-6822(87)90320-5. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Whittaker P. A., Campbell A. J., Southern E. M., Murray N. E. Enhanced recovery and restriction mapping of DNA fragments cloned in a new lambda vector. Nucleic Acids Res. 1988 Jul 25;16(14B):6725–6736. doi: 10.1093/nar/16.14.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]