Abstract
A bacterial glucoamylase was purified from the anaerobic thermophilic bacterium Clostridium thermosaccharolyticum and characterized. The enzyme, which was purified 63-fold, with a yield of 36%, consisted of a single subunit with an apparent molecular mass of 75 kDa. The purified enzyme was able to attack α-1,4- and α-1,6-glycosidic linkages in various α-glucans, liberating glucose with a β-anomeric configuration. The purified glucoamylase, which was optimally active at 70°C and pH 5.0, attacked preferentially polysaccharides such as starch, glycogen, amylopectin, and maltodextrin. The velocity of oligosaccharide hydrolysis decreased with a decrease in the size of the substrate. The Km values for starch and maltose were 18 mg/ml and 20 mM, respectively. Enzyme activity was not significantly influenced by Ca2+, EDTA, or α- or β-cyclodextrins.
Full text
PDF

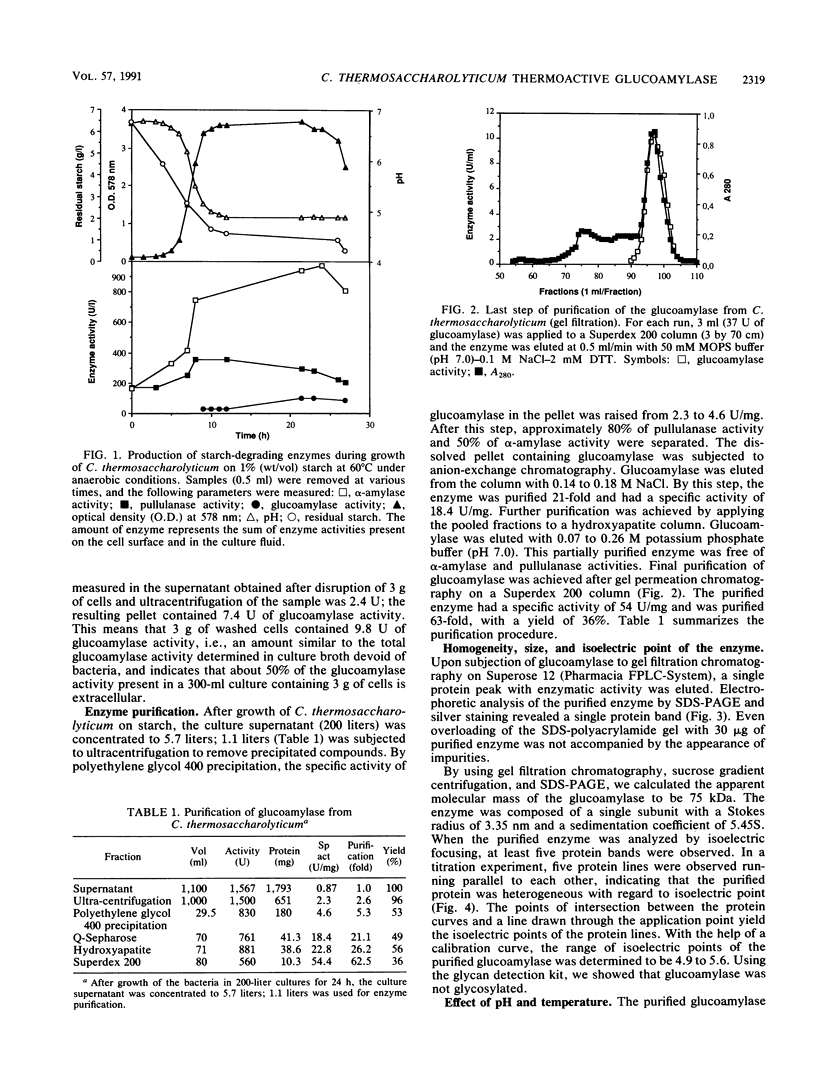
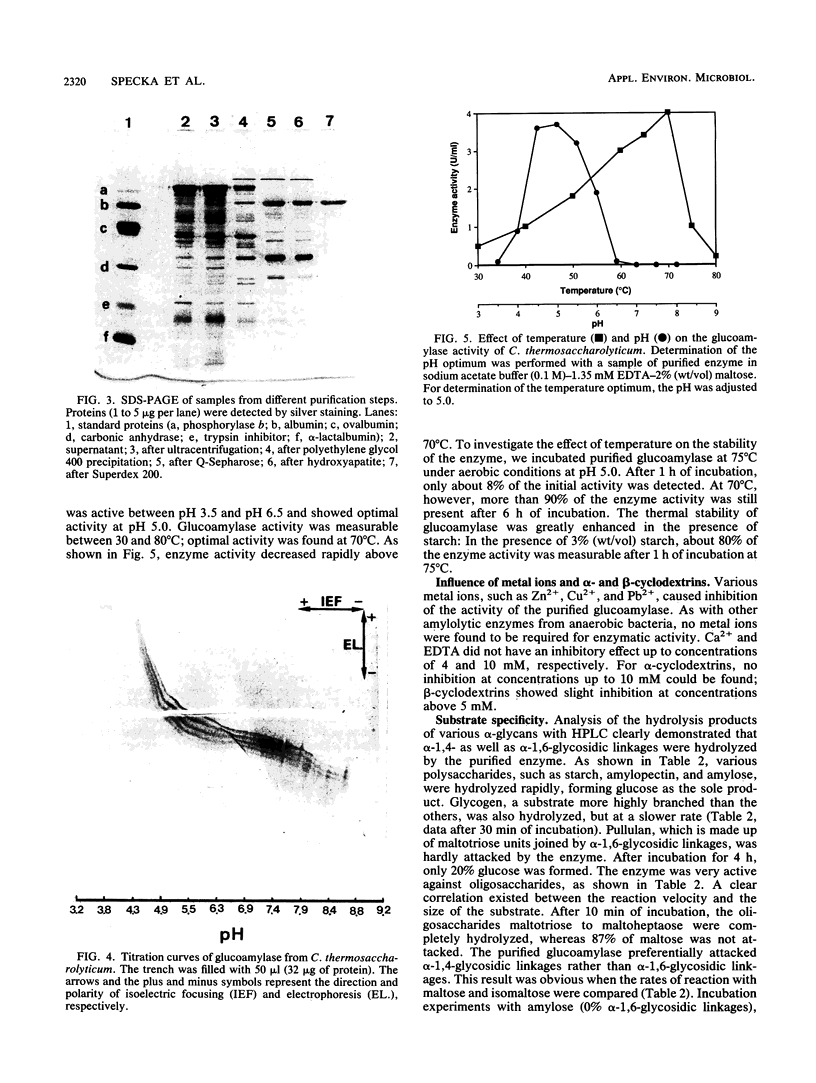
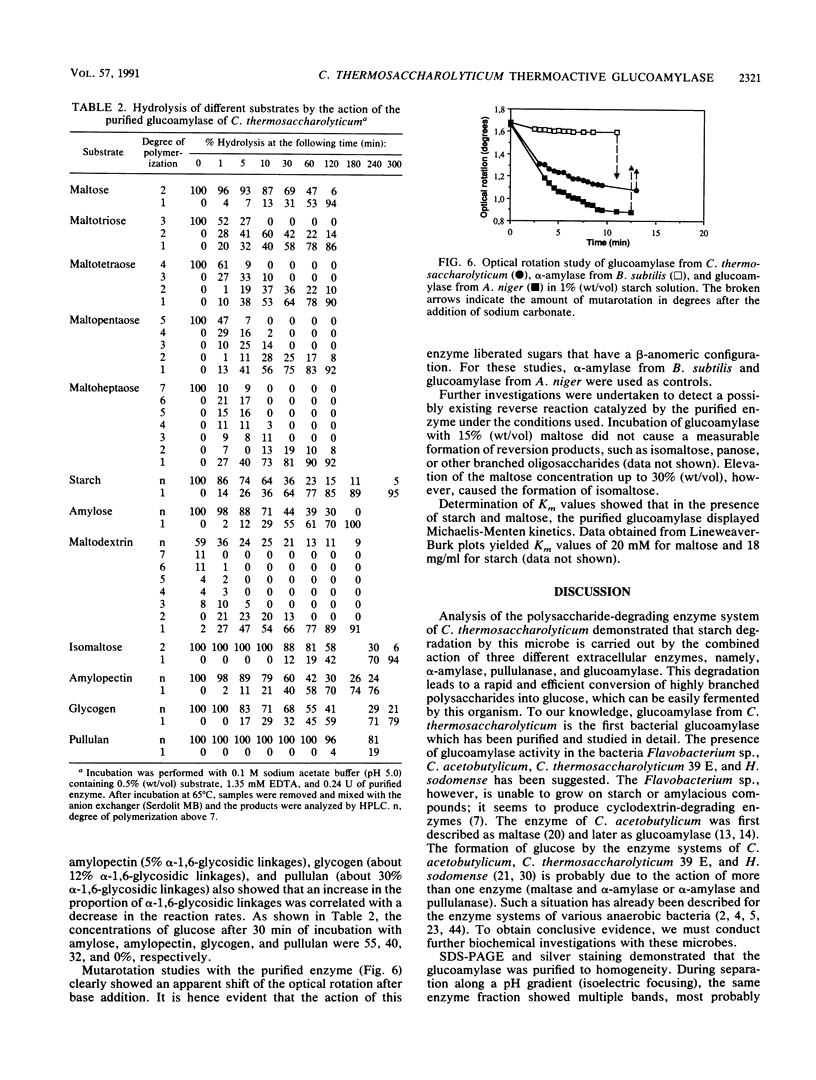


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. -O., Borg H., Mikaelsson M. Molecular weight estimations of proteins by electrophoresis in polyacrylamide gels of graded porosity. FEBS Lett. 1972 Feb 1;20(2):199–202. doi: 10.1016/0014-5793(72)80793-2. [DOI] [PubMed] [Google Scholar]
- Antranikian G., Herzberg C., Gottschalk G. Production of Thermostable alpha-Amylase, Pullulanase, and alpha-Glucosidase in Continuous Culture by a New Clostridium Isolate. Appl Environ Microbiol. 1987 Jul;53(7):1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender H. A bacterial glucoamylase degrading cyclodextrins. Partial purification and properties of the enzyme from a Flavobacterium species. Eur J Biochem. 1981 Apr;115(2):287–291. doi: 10.1111/j.1432-1033.1981.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Celis R. T. Phosphorylation in vivo and in vitro of the arginine-ornithine periplasmic transport protein of Escherichia coli. Eur J Biochem. 1984 Dec 3;145(2):403–411. doi: 10.1111/j.1432-1033.1984.tb08568.x. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G., Nikaido K. Phosphate-containing proteins of Salmonella typhimurium and Escherichia coli. Analysis by a new two-dimensional gel system. Eur J Biochem. 1981 Apr;115(3):525–531. [PubMed] [Google Scholar]
- Hockenhull D. J., Herbert D. The amylase and maltase of Clostridium acetobutylicum. Biochem J. 1945;39(1):102–106. doi: 10.1042/bj0390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Extracellular beta-Amylase from Clostridium thermosulfurogenes. Appl Environ Microbiol. 1985 May;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luthe D. S. A simple technique for the preparation and storage of sucrose gradients. Anal Biochem. 1983 Nov;135(1):230–232. doi: 10.1016/0003-2697(83)90755-8. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazur J. H., Knull H. R., Cepure A. Glycoenzymes: structure and properties of the two forms of glucoamylase from Aspergillus niger. Carbohydr Res. 1971 Nov;20(1):83–96. doi: 10.1016/s0008-6215(00)84951-4. [DOI] [PubMed] [Google Scholar]
- Saha B. C., Mathupala S. P., Zeikus J. G. Purification and characterization of a highly thermostable novel pullulanase from Clostridium thermohydrosulfuricum. Biochem J. 1988 Jun 1;252(2):343–348. doi: 10.1042/bj2520343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Inokuchi N., Irie M. Purification and characterization of a glucoamylase from Aspergillus saitoi. J Biochem. 1981 Jan;89(1):125–134. doi: 10.1093/oxfordjournals.jbchem.a133172. [DOI] [PubMed] [Google Scholar]
- Vihinen M., Mäntsälä P. Microbial amylolytic enzymes. Crit Rev Biochem Mol Biol. 1989;24(4):329–418. doi: 10.3109/10409238909082556. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Hartman P. A. Purification and some properties of an extracellular maltase from Bacillus subtilis. Appl Environ Microbiol. 1976 Jan;31(1):108–118. doi: 10.1128/aem.31.1.108-118.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]