Abstract
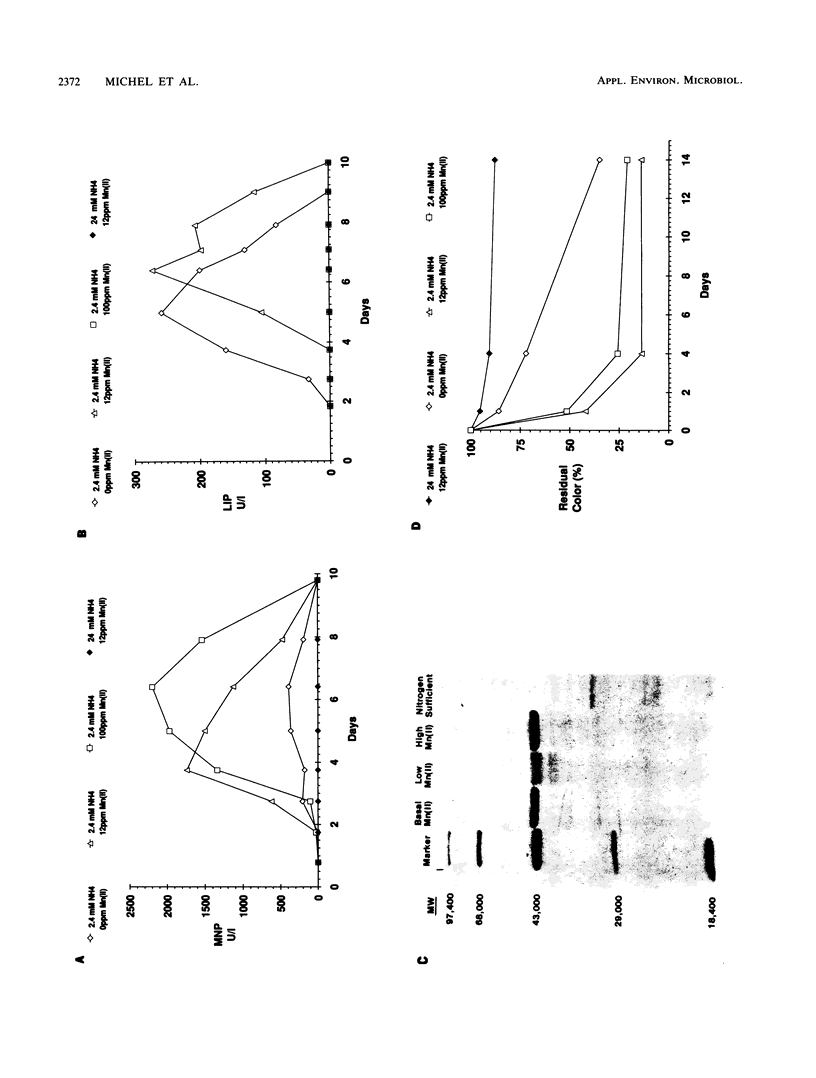
The role of lignin peroxidases (LIPs) and manganese peroxidases (MNPs) of Phanerochaete chrysosporium in decolorizing kraft bleach plant effluent (BPE) was investigated. Negligible BPE decolorization was exhibited by a per mutant, which lacks the ability to produce both the LIPs and the MNPs. Also, little decolorization was seen when the wild type was grown in high-nitrogen medium, in which the production of LIPs and MNPs is blocked. A lip mutant of P. chrysosporium, which produces MNPs but not LIPs, showed about 80% of the activity exhibited by the wild type, indicating that the MNPs play an important role in BPE decolorization. When P. chrysosporium was grown in a medium with 100 ppm of Mn(II), high levels of MNPs but no LIPs were produced, and this culture also exhibited high rates of BPE decolorization, lending further support to the idea that MNPs play a key role in BPE decolorization. When P. chrysosporium was grown in a medium with no Mn(II), high levels of LIPs but negligible levels of MNPs were produced and the rate and extent of BPE decolorization by such cultures were quite low, indicating that LIPs play a relatively minor role in BPE decolorization. Furthermore, high rates of BPE decolorization were seen on days 3 and 4 of incubation, when the cultures exhibit high levels of MNP activity but little or no LIP activity. These results indicate that MNPs play a relatively more important role than LIPs in BPE decolorization by P. chrysosporium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Kelley R. L., Reddy C. A. Lignin peroxidase-negative mutant of the white-rot basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1990 Jan;172(1):260–265. doi: 10.1128/jb.172.1.260-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass S. B., Reddy C. A. Characterization of extracellular peroxidases produced by acetate-buffered cultures of the lignin-degrading basidiomycete Phanerochaete chrysosporium. FEMS Microbiol Lett. 1990 Jun 1;57(3):221–224. doi: 10.1111/j.1574-6968.1990.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Dosoretz C. G., Dass S. B., Reddy C. A., Grethlein H. E. Protease-mediated degradation of lignin peroxidase in liquid cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Nov;56(11):3429–3434. doi: 10.1128/aem.56.11.3429-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Lamar R. T., Larsen M. J., Kirk T. K. Sensitivity to and Degradation of Pentachlorophenol by Phanerochaete spp. Appl Environ Microbiol. 1990 Nov;56(11):3519–3526. doi: 10.1128/aem.56.11.3519-3526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]





