Abstract
Import of most nucleus-encoded preproteins into mitochondria is mediated by N-terminal presequences and requires a membrane potential and ATP hydrolysis. Little is known about the chemical nature and localization of other mitochondrial targeting signals or of the mechanisms by which they facilitate membrane passage. Mitochondrial heme lyases lack N-terminal targeting information. These proteins are localized in the intermembrane space and are essential for the covalent attachment of heme to c type cytochromes. For import of heme lyases, the translocase of the mitochondrial outer membrane complex is both necessary and sufficient. Here, we report the identification of the targeting signal of mitochondrial heme lyases in the third quarter of these proteins. The targeting sequence is highly conserved among all known heme lyases. Its chemical character is hydrophilic because of a large fraction of both positively and negatively charged amino acid residues. These features clearly distinguish this signal from classical presequences. When inserted into a cytosolic protein, the targeting sequence directs the fusion protein into the intermembrane space, even in the absence of a membrane potential or ATP hydrolysis. The heme lyase targeting sequence represents the first topogenic signal for energy-independent transport into the intermembrane space and harbors two types of information. It assures accurate recognition and translocation by the translocase of the mitochondrial outer membrane complex, and it is responsible for driving the import reaction by undergoing high-affinity interactions with components of the intermembrane space.
Targeting and translocation of most nucleus-encoded mitochondrial proteins depend on N-terminal extensions referred to as mitochondrial targeting sequences or presequences (1–3). A presequence typically consists of 15–40 amino acid residues and is enriched in positively charged and hydroxylated (mostly serine) residues. The ability of most presequence peptides to form amphipathic α-helices is thought to be important for their recognition by the translocation machineries in the mitochondrial outer (TOM complex) and inner (TIM complex) membranes (4, 5). Biochemical studies of the past few years have established a series of interactions of the translocating presequence during entry of mitochondria. At the TOM complex, these interactions are established with surface receptors (mainly Tom20 and Tom22) and the translocation channel (mainly Tom40; reviewed in refs. 6 and 7). At the TIM17/23 complex, the presequence interacts with the intermembrane space domain of Tim23 (8) before it is transferred to Tim44 and Hsp70 on the matrix side.
A significant fraction of mitochondrial proteins (about 30%) lack typical N-terminal presequences. In particular, all proteins of the mitochondrial outer membrane and some proteins of the intermembrane space and the inner membrane are devoid of such signals. For a small number of these preproteins, the targeting signals have been identified. Some outer membrane proteins such as Tom70 contain N-terminal targeting information consisting of a short presequence-like segment, followed by a membrane-anchor (“stop-transfer”) sequence (9, 10). Insertion of Tom22 into the outer membrane depends on an internal segment resembling an N-terminal presequence (11). Thus, the Tom70 and Tom22 precursors are recognized by the TOM machinery by features similar to presequence-containing preproteins (12, 13). Another class of outer membrane proteins represented by Bcl-2 carries import information within a C-terminal hydrophobic segment that mediates membrane insertion (for review see ref. 14).
At the inner membrane, the insertion of the integral protein Bcs1p by the TIM17/23 complex requires an internal presequence-like segment (15). This targeting element functions in combination with a preceding hydrophobic stretch to achieve membrane potential-dependent integration into the bilayer. Members of the family of mitochondrial carrier proteins lack N-terminal targeting information (16). Internal positively charged segments known as “carrier sequence motifs” have been proposed to act as targeting signals (17). The carrier proteins are recognized by a group of small Tim proteins in the intermembrane space, which deliver them to the Tim22/54 complex for membrane integration (see, e.g., refs. 17–21). A similar import pathway seems to be followed by several integral membrane proteins (22).
For many other mitochondrial proteins, the targeting signals and mechanisms of recognition and transport are hitherto unknown. A particularly interesting family of proteins are mitochondrial cytochrome heme lyases, which are peripherally associated with the outer face of the inner membrane (23). Cytochrome c1 heme lyase (CC1HL) and cytochrome c heme lyase (CCHL) mediate the covalent attachment of heme to the apoforms of cytochromes c1 and c, respectively (for review see refs. 24 and 25). Translocation of these proteins has been shown to follow a unique pathway (26, 27). For entry into the intermembrane space, heme lyase precursors use the TOM complex, which has been shown to be both necessary (13) and sufficient (28). The preprotein receptors Tom20 and Tom22 are required for recognition at the mitochondrial surface (29, 30). In contrast to other mitochondrial proteins of internal compartments, translocation of heme lyases occurs independently of the TIM machineries and of an electrical membrane potential (ΔΨ) at the inner membrane and does not require ATP (13, 26, 27). Despite these insights into the translocation mechanism of heme lyases, the location and nature of the targeting information contained within these proteins were unknown.
Here, we describe the identification of the import signal contained within heme lyases. By systematic introduction of deletions into the CCHL of Neurospora crassa, we were able to locate the segment that is essential for import into the intermembrane space. The targeting signal is conserved among all known heme lyases and represents a general topogenic signal for protein sorting to the intermembrane space. The chemical properties of the “heme lyase targeting signal” are distinct from those of presequences. Therefore, this sequence represents a distinct targeting signal that mediates the direct entry into the mitochondrial intermembrane space, even in the absence of an external energy supply.
Materials and Methods
General Procedures.
Mitochondria isolated from the Saccharomyces cerevisiae strain W303a (MATa, ura3-1, ade2-1, trp1-1, his3-11,15, leu2-3,112) were employed for in vitro protein import experiments. Cells were grown as detailed previously in rich medium containing 2% (vol/vol) galactose (31, 32). Standard procedures for the manipulation of DNA and for PCR were used (33). The following published methods were employed: isolation of yeast mitochondria (34); purification of mitochondria by Nycodenz density gradient centrifugation (35); in vitro transcription and translation and protein import into isolated or density gradient-purified mitochondria (13, 27, 36, 37); subfractionation of mitochondria by hypotonic treatment (38); and depletion of ATP and the membrane potential (26, 27).
Plasmid Constructions.
The coding region of N. crassa CCHL (39) was subcloned in the vector pGEM-4Z as a PCR fragment by using the EcoRI/BamHI sites. This plasmid was used as a template for PCRs to synthesize partial regions of the CCHL gene. These DNA fragments were then inserted into pGEM-4Z to result in DNA segments that encode the mutant CCHL proteins carrying the desired deletions. For N- and C-terminal truncations (CCHL Δ2–70, CCHL Δ2–170, CCHL Δ177–346, CCHL Δ281–346, and CCHL Δ2–155;Δ280–346 mutant proteins), PCR products corresponding to the remaining coding regions of CCHL with flanking EcoRI and XbaI restriction sites were synthesized and inserted into the corresponding sites of pGEM-4Z. Internal deletion constructs (CCHL Δ83–170, CCHL Δ177–273, CCHL Δ157–176, CCHL Δ177–202, CCHL Δ204–241, and CCHL Δ243–276) were produced in two steps. First, the DNA fragments corresponding to the C-terminal pieces were synthesized as BglII/XbaI PCR products, digested with XbaI, and inserted into the SmaI/XbaI sites of pGEM-4Z. Then, the N-terminal fragments were ligated into this plasmid as EcoRI/BglII PCR products.
The plasmids encoding the fusion proteins of dihydrofolate reductase (DHFR) and CCHL (termed DCD 203–225 and DCD 171–279) were obtained by inserting the entire or the N-terminal half of mouse DHFR into pGEM-4Z. PCR fragments of the N-terminal half of DHFR flanked by EcoRI/BglII sites were introduced into the EcoRI/SmaI sites of the vector. The DNA segments representing residues 203–225 and 171–279 of CCHL were inserted into these plasmids as BglII/XbaI fragments. Finally, the C-terminal portion of DHFR was inserted by using the XbaI/HindIII sites.
The fusion protein DC1D 101–169 was created by inserting amino acid residues 101–169 of S. cerevisiae CC1HL (40) into the middle of DHFR. For generation of the coding sequence of this protein, the pGEM-4Z plasmid carrying the coding region of DCD 171–279 was cleaved with BglII and XbaI restriction enzymes to remove the N. crassa CCHL-derived fragment. A PCR fragment corresponding to codons 101–169 of S. cerevisiae CC1HL was inserted into the BglII/XbaI sites of the residual DNA fragment and used for transcription of the encoded fusion protein.
Results
To identify the import signal in mitochondrial heme lyases, we chose CCHL of N. crassa as a model protein, introduced a series of deletions into its coding sequence, and studied the import of the mutant proteins into isolated mitochondria. First, mutant CCHL constructs were generated corresponding to the coding regions of either the N- or C-terminal halves of the protein. The mutant proteins were synthesized by in vitro transcription and translation in reticulocyte lysate in the presence of radioactive [35S]methionine. An import reaction was performed by using isolated mitochondria, and translocation was assessed by treatment of the samples with proteinase K (Fig. 1). Import was quantitated by PhosphorImager analysis from the amount of protease-resistant preprotein relative to bound material. The correct location of the imported mutant proteins in the intermembrane space was estimated by selectively opening the outer membrane by hypotonic treatment (swelling) of the mitochondria (see Fig. 1D). Residual material resistant to treatment by proteinase K under these conditions represented aggregated protein.
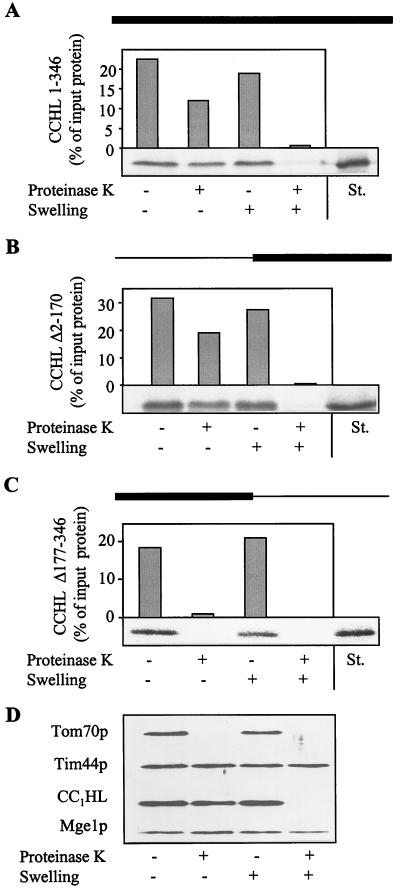
Figure 1.
CCHL does not contain essential import information in its N-terminal half. The precursor proteins CCHL (A), CCHL Δ2–170 (B), and CCHL Δ177–346 (C; see Materials and Methods) were synthesized by in vitro transcription and translation in reticulocyte lysate by using [35S]methionine as a label. The black bar on top of each panel represents the region of CCHL present in these proteins; the thin line corresponds to deleted segments. The radiolabeled proteins were added to isolated mitochondria in import buffer (36). After incubation for 10 min at 25°C, mitochondria were reisolated by centrifugation (10 min at 9,000 × g). Mitochondria were resuspended in a small volume of SoH buffer (0.6 M sorbitol/20 mM Hepes-KOH, pH 7.2). Samples were diluted 10-fold into SoH buffer or water in the presence or absence of proteinase K as indicated. The hypotonic condition results in swelling of the organelles and leads to selective rupture of the outer membrane allowing added proteinase K to degrade proteins exposed in the intermembrane space (D, CC1HL) but not of proteins of the matrix (Tim44p and Mge1p). After incubation for 30 min at 0°C, protease digestion was halted by the addition of PMSF, and proteins were precipitated with trichloroacetic acid. Proteins were separated by SDS/PAGE, blotted on nitrocellulose, and quantified by PhosphorImager analysis. In addition, an autoradiograph is shown. The material that is not digested after swelling represents aggregated preprotein. The rightmost lanes contain 50% of input preprotein as a standard (St.). Import (i.e., protease-resistant protein relative to bound material) varied by not more than 15% in various experiments.
The C-terminal portion of CCHL (CCHL Δ2–170) showed high import efficiency, which was comparable to that of intact CCHL (Fig. 1 A and B). Relative to the amount bound to mitochondria, at least 50% became protease-resistant indicating efficient import of the C-terminal half of CCHL into the intermembrane space. In contrast, no import was observed with the N-terminal half of CCHL (CCHL Δ177–346; Fig. 1C). We conclude that there is no essential import information contained within the N-terminal region of CCHL, distinguishing this protein from typical presequence-containing preproteins. Conversely, the C-terminal half of the protein harbors the signals required for efficient translocation into mitochondria.
Next, we constructed mutant proteins in which quarters of the CCHL sequence were deleted. The in vitro import experiments revealed that the third quarter was necessary to direct CCHL into the intermembrane space, whereas deletion of the other three quarters had little or no effect on the efficiency of import (Fig. 2 A–D). These data are consistent with the results shown in Fig. 1 and suggest that the targeting information within CCHL is not scattered throughout the protein sequence but rather is confined to a short region in the third quarter of the protein.
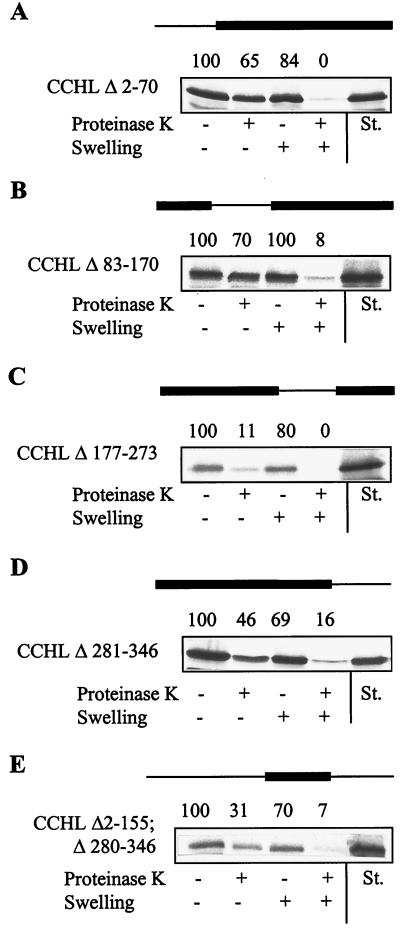
Figure 2.
The third quarter of CCHL contains both necessary and sufficient targeting information. The import of the indicated CCHL mutant proteins was estimated as described in the legend of Fig. 1. The amount of radiolabeled protein was quantitated by PhosphorImager analysis and is given above the autoradiographs relative to the amount of preprotein bound to mitochondria (set to 100%). The rightmost lanes contain 50% of input preprotein (St., standard).
Is the targeting information located in the third quarter of CCHL sufficient to support import into the intermembrane space? To address this question, we constructed four mutant proteins that consisted of each of the quarters of CCHL. Efficient import was observed only for CCHL Δ2–155–Δ280–346, i.e., the third quarter of CCHL (Fig. 2E), whereas the other quarters did not become translocated (not shown). Together, the data presented in Fig. 2 show that sequence contained within the third quarter of CCHL is both necessary and sufficient for translocation into the intermembrane space.
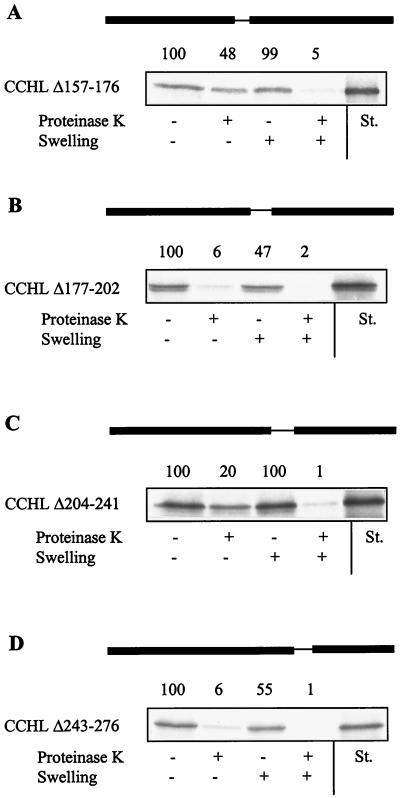
To narrow down the region responsible for targeting, small deletions of about 30 amino acid residues were introduced into the third quarter of CCHL. Mutant heme lyase proteins lacking either residues 177–202 (CCHL Δ177–202) or 243–276 (CCHL Δ243–276) became imported into isolated mitochondria only weakly, whereas wild-type import efficiency was observed for CCHL Δ157–176 in which a segment near the N terminus of the third quarter was deleted (Fig. 3). Deletion of the residues 204–241 (CCHL Δ204–241) decreased the import efficiency about 2.5-fold compared with that of native CCHL. Most of this segment is not conserved in other heme lyases and represents a sequence present only in N. crassa CCHL (ref. 41; see Fig. 6). The segments responsible for efficient import (residues 177–202 and 243–276) contain motifs that are highly conserved in all known heme lyases, suggesting that the identified sequences represent a general signal for targeting of these proteins.
Figure 3.
The import information of N. crassa CCHL is contained within two conserved small segments. Import of the indicated CCHL mutant proteins was estimated and analyzed as described in the legend of Fig. 2. St., standard representing 50% of input preprotein.
Figure 6.
The heme lyase targeting signal encompasses two signature motifs that are highly conserved in these proteins. The sequence alignment of known heme lyases was prepared by using the multalin program (54). No bacterial homologues have been identified. The heme lyase targeting signal determined in this study is underlined. The secondary structure of these sequences was predicted by using the phdsec program (55) revealing similar results for CCHL and CC1HL proteins. H, α-helical; E, extended β-stranded. Nc, N. crassa; Sp, Schizosaccharomyces pombe; Sc, S. cerevisiae; Ca, Candida albicans; Hs, Homo sapiens; Mm, Mus musculus; Ce, Caenorhabditis elegans.
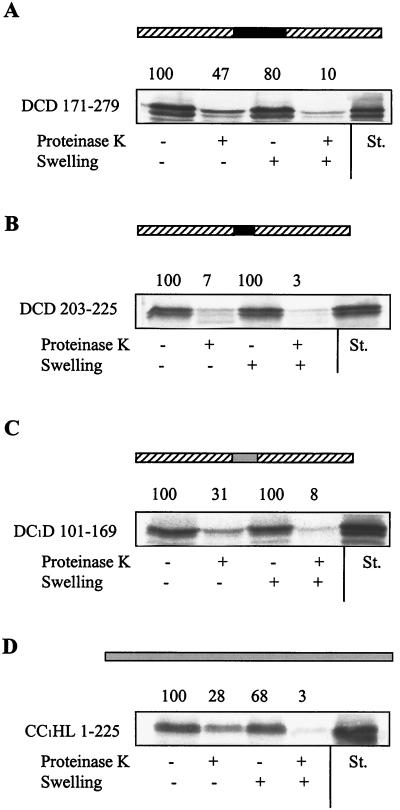
To investigate whether the targeting signal of CCHL can support the translocation of passenger proteins into the intermembrane space, we inserted residues 171–279 into the cytosolic protein DHFR. The fusion protein DCD 171–279 became translocated into the mitochondrial intermembrane space at efficiencies similar to those of native CCHL (Fig. 4A). In contrast, no import was observed when other segments of CCHL, e.g., the sequence present only in N. crassa CCHL (fusion protein DCD 203–225), were introduced into DHFR (Fig. 4B and data not shown). Likewise, DHFR did not become transported, even after denaturation of this protein before the import reaction (not shown). Interestingly, when attached to the N or C terminus of DHFR, the heme lyase targeting signal could not support import (not shown). The reason for this observation is unknown, but one might speculate that translocation of heme lyases involves a looped structure. Taken together, the results of Figs. 3 and 4 A and B suggest that the conserved segments comprised by amino acid residues 177–202 and 243–276 of N. crassa CCHL harbor the information that is essential and sufficient for import into the mitochondrial intermembrane space.
Figure 4.
The targeting sequence of heme lyases can direct a cytosolic protein into the intermembrane space. The indicated regions of N. crassa CCHL (A and B) or of S. cerevisiae CC1HL (C) were inserted into the cytosolic protein DHFR. Import of these fusion proteins or of CC1HL (D) and further analysis of import were performed as described in the legend of Fig. 2. Other parts of the heme lyases did not support the import of the corresponding fusion proteins (not shown). The DHFR sequence is indicated by the hatched boxes; the N. crassa CCHL and S. cerevisiae CC1HL sequences are given as black and grey bars, respectively. St., standard representing 50% of input preprotein.
The conserved nature of the targeting signal identified in N. crassa CCHL suggests that this sequence also may be used by other proteins, including CC1HL, for sorting to the intermembrane space. To test this expectation, the respective sequence of S. cerevisiae CC1HL was inserted into DHFR, and the import of the fusion protein DC1D 101–169 was followed. DC1D 101–169 was imported into the intermembrane space at an efficiency comparable to translocation of CC1HL (Fig. 4 C and D). This result provides convincing evidence that this conserved segment of heme lyases is both necessary and sufficient for import into mitochondria and serves as a general targeting signal.
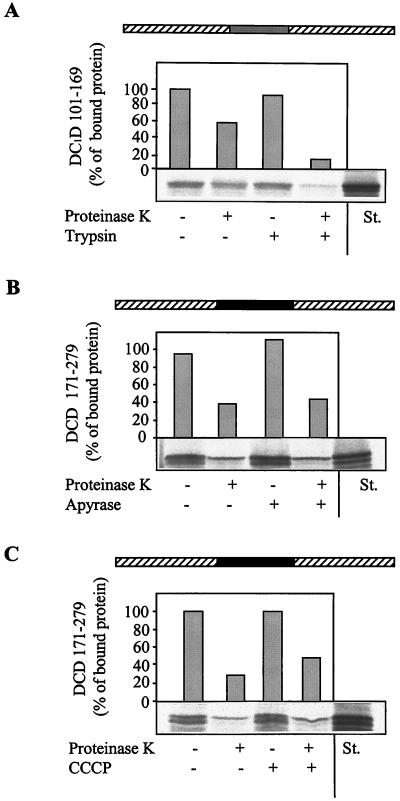
We finally asked whether the identified targeting sequence carries attached passenger proteins along the authentic import pathway of heme lyases. To this end, we investigated the requirement of protease-sensitive surface receptors and external energy sources for the import of DCD 171–279 and DC1D 101–169. Translocation of both DHFR fusion proteins was strongly impaired after trypsin treatment of the mitochondria before the import reaction (Fig. 5A and data not shown). This impairment suggests the involvement of the surface receptors of the TOM complex in transport of these proteins, because a similar impairment has been reported for intact heme lyases (29, 30).
Figure 5.
Import mediated by the heme lyase targeting sequence occurs along the authentic heme lyase import pathway. The fusion proteins DCD 171–279 and DC1D 101–169 were imported into isolated mitochondria. (A) The organelles were pretreated with 50 μg/ml trypsin (27). (B) ATP was hydrolyzed by treatment with 20 units/ml apyrase (ref. 27; import reactions contained 20 μM oligomycin and 5 μM carboxy-atractyloside). (C) The membrane potential was depleted by the addition of the uncoupling reagent carbonyl cyanide m-chlorophenylhydrazone (50 μM; CCCP; ref. 26). Under any of these conditions, import of presequence-containing preproteins into the matrix was strongly impaired (not shown). Further treatment of the samples and analysis of import were performed as described in Fig. 2. The result for only one of the heme lyase/DHFR fusion proteins is shown.
Further, the dependence of transport of the DHFR fusion proteins on external energy sources was analyzed by employing isolated mitochondria in which either ATP was depleted by apyrase treatment or the membrane potential (ΔΨ) was uncoupled by the addition of carbonyl cyanide m-chlorophenylhydrazone. The lack of ATP or ΔΨ did not influence the import of DCD 171–279 or DC1D 101–169 fusion proteins significantly (Fig. 5 B and C and data not shown). Thus, heme lyase targeting sequences carry attached proteins into the intermembrane space without a requirement for external energy sources. Apparently, these sequences not only harbor the information for correct localization but also contain the potential to drive the import reaction. In conclusion, the targeting sequence of heme lyases can direct passenger proteins into the intermembrane space along the authentic pathway of heme lyases.
Discussion
In this report, we have identified the sequence element responsible for sorting of mitochondrial heme lyases to the intermembrane space. The heme lyase targeting signal represents a distinct topogenic sequence that differs structurally and functionally from classical N-terminal presequences. It is located in the third quarter of the heme lyase molecules and comprises about 60 amino acid residues. The sequence contains two highly conserved motifs that are characteristic signatures of all known mitochondrial heme lyases (Fig. 6; ref. 42). It is conceivable that these regions, in addition to their role in sorting, are essential for the proper function of the proteins in heme attachment. The targeting signal is both necessary and sufficient for entry into the intermembrane space. It represents a general topogenic signal, because it possesses the capacity to direct even nonmitochondrial proteins to this compartment with high efficiency. In this aspect, the heme lyase targeting signal behaves similarly to classical mitochondrial presequences that are capable of targeting attached proteins to the mitochondrial matrix (43).
Several properties distinguish the heme lyase targeting signal from mitochondrial presequences. The latter can pass across both mitochondrial membranes, whereas the heme lyase targeting signal is capable of guiding proteins only across the outer membrane. Nevertheless, sorting of heme lyases to the intermembrane space can be overridden when a presequence is attached to the N terminus of the protein (44). The N-terminal targeting signal causes the membrane potential-dependent entry of the fusion protein into the matrix. Presequences, in contrast to the heme lyase signal, do not have any sequence homology, not even when orthologous proteins of different species are compared. This fact suggests a specific interaction of the heme lyase signal with a component of the target compartment (see below). The heme lyase targeting signal is highly hydrophilic (30% charged residues) containing a similar number of positively and negatively charged residues that are distributed over the entire sequence element. Secondary structure predictions for this sequence assign a helical structure to the regions corresponding to residues 184–198 and 249–259 of N. crassa CCHL and an extended structure for residues 275–278. No structural preference is revealed for the remainder portions (Fig. 6). Presequences usually do not harbor negative charges and are predicted to form amphipathic helices (4, 5). Thus, the chemical character of the heme lyase signal differs markedly from that of presequences. From all these criteria, it is evident that the heme lyase targeting signal represents a distinct topogenic sequence with the capacity to guide proteins to the intermembrane space.
A comparison of the mechanisms by which presequences and the heme lyase targeting signal mediate translocation of attached proteins reveals striking similarities for transport across the outer membrane but substantial differences for passage across the inner membrane. At the surface of the outer membrane, the presequence is recognized by the receptors Tom20 and Tom22 (45–47). From this “cis-binding site,” the presequence is reversibly transferred across an aqueous channel comprised of Tom40 (28, 48) to reach the “trans site” that is formed by intermembrane space regions of Tom40 (45, 49, 50). Our data together with previous results indicate a similar mechanism for transport mediated by the heme lyase targeting signal involving binding to at least two sites on either face of the outer membrane. At the cis side, heme lyases interact with the surface receptors Tom20 and Tom22 (29, 30). The different chemical properties of presequences and the heme lyase targeting signal make it likely that distinct regions on the receptor proteins are used for interaction with these signals at the mitochondrial surface. The ability of the receptors to differentiate between various portions of targeting signals has been reported recently (51). These findings imply multiple binding regions on the preprotein receptors and raise the question of their precise location. It will also be interesting to define the types of forces engaged between presequences, the heme lyase targeting signal, and receptors.
A trans-binding site for heme lyases seems to be located at the inner face of the TOM complex, because purified outer membrane vesicles or proteoliposomes containing only isolated TOM complex support the import of CCHL (13, 28). Presumably, Tom40 participates in this specific interaction as it does in binding of presequences. Further, a component at the outer face of the inner membrane may be responsible for the release of heme lyases from the TOM complex and their transfer to the inner membrane, the functional site of these proteins (23). It is unknown how this transfer occurs, but it is tempting to speculate that the targeting sequence may also play a role in assuring exclusive localization at the inner membrane.
Apart from the selective interaction of the presequence with the intermembrane space domain of Tim23 (8), an entirely different mechanism is used for translocation of the presequence across the mitochondrial inner membrane. Transfer of the presequence into the matrix does not occur spontaneously but is driven by an electrochemical potential (52). Presumably, this positively charged signal uses an electrophoretic mechanism to slide through the membrane (53) and reach Tim44 and mitochondrial Hsp70, which are not specific for presequences. Translocation of the other parts of the preprotein requires the ATP-dependent function of mitochondrial Hsp70. Thus, the energetic features of membrane passage discriminate the transport of heme lyases and presequence-containing preproteins.
The import mechanism unraveled for heme lyases provides a paradigm for a pathway that does not require an external energy input. Both ATP and a membrane potential at the inner membrane are dispensable for membrane passage of heme lyases (refs. 26 and 27 and this study). Previously, it has been shown that the energy of protein folding in the intermembrane space cannot account for the efficient and specific localization to the intermembrane space (27). The data reported here further support this conclusion. These findings raise the question of what drives the import of heme lyases. All available evidence suggests that entry into mitochondria is propelled by high-affinity interactions of the heme lyase targeting sequence with components of the intermembrane space, e.g., by internal regions of Tom40 (see above). In this view, the topogenic signal identified by our work harbors two types of information that are essential for localization of attached proteins to the intermembrane space. First, the targeting signal determines the specificity of transport into this compartment. Second, the signal is responsible for driving the import reaction, presumably by undergoing one or more high-affinity interactions with components of the intermembrane space.
The identification of the internal import signal in heme lyases will now facilitate investigations on its precise molecular function and on the structural and energetic basis for its interaction with components of the import machinery.
Acknowledgments
This paper is dedicated to Prof. Walter Neupert on the occasion of his 60th birthday. The expert technical assistance of M. Dienst and B. Niggemeyer is gratefully acknowledged. Our work was supported by grants of the Sonderforschungsbereich 286 of the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, and the Alexander von Humboldt-Stiftung.
Abbreviations
- TOM
translocase of the mitochondrial outer membrane
- TIM
translocase of the mitochondrial inner membrane
- CCHL
cytochrome c heme lyase
- CC1HL
cytochrome c1 heme lyase
- DHFR
dihydrofolate reductase
References
- 1.Neupert W. Annu Rev Biochem. 1997;66:861–915. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 2.Schatz G, Dobberstein B. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 3.Pfanner N, Craig E A, Honlinger A. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- 4.von Heijne G. EMBO J. 1986;5:1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roise D, Schatz G. J Biol Chem. 1988;263:4509–4511. [PubMed] [Google Scholar]
- 6.Lill R, Neupert W. Trends Cell Biol. 1996;6:56–61. doi: 10.1016/0962-8924(96)81015-4. [DOI] [PubMed] [Google Scholar]
- 7.Lill R, Nargang F E, Neupert W. Curr Opin Cell Biol. 1996;8:505–512. doi: 10.1016/s0955-0674(96)80028-7. [DOI] [PubMed] [Google Scholar]
- 8.Bauer M F, Sirrenberg C, Neupert W, Brunner M. Cell. 1996;87:33–41. doi: 10.1016/s0092-8674(00)81320-3. [DOI] [PubMed] [Google Scholar]
- 9.Hurt E C, Müller U, Schatz G. EMBO J. 1985;4:3509–3518. doi: 10.1002/j.1460-2075.1985.tb04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride H M, Millar D G, Li J M, Shore G C. J Cell Biol. 1992;119:1451–1457. doi: 10.1083/jcb.119.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Cousino N, Nargang F E, Baardman R, Neupert W, Lill R, Court D A. J Biol Chem. 1998;273:11527–11532. doi: 10.1074/jbc.273.19.11527. [DOI] [PubMed] [Google Scholar]
- 12.Keil P, Pfanner N. FEBS Lett. 1993;321:197–200. doi: 10.1016/0014-5793(93)80107-6. [DOI] [PubMed] [Google Scholar]
- 13.Mayer A, Lill R, Neupert W. J Cell Biol. 1993;121:1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shore G C, McBride H M, Millar D G, Steenaart N A M, Nguyen M. Eur J Biochem. 1995;227:9–18. doi: 10.1111/j.1432-1033.1995.tb20354.x. [DOI] [PubMed] [Google Scholar]
- 15.Fölsch H, Guiard B, Neupert W, Stuart R A. EMBO J. 1996;15:479–487. [PMC free article] [PubMed] [Google Scholar]
- 16.Pfanner N, Hoeben P, Tropschug M, Neupert W. J Biol Chem. 1987;262:14851–14854. [PubMed] [Google Scholar]
- 17.Sirrenberg C, Endres M, Folsch H, Stuart R A, Neupert W, Brunner M. Nature (London) 1998;391:912–915. doi: 10.1038/36136. [DOI] [PubMed] [Google Scholar]
- 18.Kerscher O, Holder J, Srinivasan M, Leung R S, Jensen R E. J Cell Biol. 1997;139:1663–1675. doi: 10.1083/jcb.139.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehler C M, Jarosch E, Tokatlidis K, Schmid K, Schweyen R J, Schatz G. Science. 1998;279:369–373. doi: 10.1126/science.279.5349.369. [DOI] [PubMed] [Google Scholar]
- 20.Adam A, Endres M, Sirrenberg C, Lottspeich F, Neupert W, Brunner M. EMBO J. 1999;18:313–319. doi: 10.1093/emboj/18.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehler C M, Leuenberger D, Merchant S, Renold A, Junne T, Schatz G. Proc Natl Acad Sci USA. 1999;96:2141–2146. doi: 10.1073/pnas.96.5.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaldi K, Bauer M F, Sirrenberg C, Neupert W, Brunner M. EMBO J. 1998;17:1569–1576. doi: 10.1093/emboj/17.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lill R, Kispal G, Künkele K P, Mayer A, Risse B, Steiner H, Heckmeyer P, Van der Klei I, Court D A. In: Proceedings of the NATO/ASI, Cell Biology: Molecular Dynamics of Biomembranes. Op den Kamp J A F, editor. H. Berlin: Springer; 1996. pp. 137–155. [Google Scholar]
- 24.Dumont M E. In: Advances in Molecular and Cell Biology. Hartl F U, editor. Vol. 17. Greenwich: JAI Press; 1996. pp. 103–126. [Google Scholar]
- 25.Kranz R, Lill R, Goldman B, Bonnard G, Merchant S. Mol Microbiol. 1998;29:383–396. doi: 10.1046/j.1365-2958.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- 26.Lill R, Stuart R A, Drygas M E, Nargang F E, Neupert W. EMBO J. 1992;11:449–456. doi: 10.1002/j.1460-2075.1992.tb05074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiner H, Zollner A, Haid A, Neupert W, Lill R. J Biol Chem. 1995;270:22842–22849. doi: 10.1074/jbc.270.39.22842. [DOI] [PubMed] [Google Scholar]
- 28.Künkele K P, Heins S, Dembowski M, Nargang F E, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. Cell. 1998;93:1009–1019. doi: 10.1016/s0092-8674(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 29.Harkness T A A, Nargang F E, Van der Klei I, Neupert W, Lill R. J Cell Biol. 1994;124:637–648. doi: 10.1083/jcb.124.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nargang F E, Künkele K-P, Mayer A, Ritzel R G, Neupert W, Lill R. EMBO J. 1995;14:1099–1108. doi: 10.1002/j.1460-2075.1995.tb07093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kispal G, Csere P, Guiard B, Lill R. FEBS Lett. 1997;418:346–350. doi: 10.1016/s0014-5793(97)01414-2. [DOI] [PubMed] [Google Scholar]
- 32.Sherman F. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 34.Daum G, Böhni P C, Schatz G. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- 35.Lewin A S, Hines V, Small G M. Mol Cell Biol. 1990;10:1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glick B S. Methods Cell Biol. 1991;34:389–397. doi: 10.1016/s0091-679x(08)61693-3. [DOI] [PubMed] [Google Scholar]
- 37.Söllner T, Rassow J, Pfanner N. Methods Cell Biol. 1991;34:345–358. doi: 10.1016/s0091-679x(08)61689-1. [DOI] [PubMed] [Google Scholar]
- 38.Glick B S. Methods Enzymol. 1995;260:224–231. doi: 10.1016/0076-6879(95)60140-6. [DOI] [PubMed] [Google Scholar]
- 39.Drygas M E, Lambowitz A M, Nargang F E. J Biol Chem. 1989;264:17897–17907. [PubMed] [Google Scholar]
- 40.Zollner A, Rödel G, Haid A. Eur J Biochem. 1992;207:1093–1100. doi: 10.1111/j.1432-1033.1992.tb17146.x. [DOI] [PubMed] [Google Scholar]
- 41.Steiner H, Kispal G, Zollner A, Haid A, Neupert W, Lill R. J Biol Chem. 1996;271:32605–32611. doi: 10.1074/jbc.271.51.32605. [DOI] [PubMed] [Google Scholar]
- 42.Bairoch A, Bucher P, Hofmann K. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurt E C, Pesold-Hurt B, Schatz G. EMBO J. 1984;3:3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segui-Real B, Kispal G, Lill R, Neupert W. EMBO J. 1993;12:2211–2218. doi: 10.1002/j.1460-2075.1993.tb05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer A, Neupert W, Lill R. Cell. 1995;80:127–137. doi: 10.1016/0092-8674(95)90457-3. [DOI] [PubMed] [Google Scholar]
- 46.Bolliger L, Junne T, Schatz G, Lithgow T. EMBO J. 1995;14:6318–6326. doi: 10.1002/j.1460-2075.1995.tb00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brix J, Dietmeier K, Pfanner N. J Biol Chem. 1997;272:20730–20735. doi: 10.1074/jbc.272.33.20730. [DOI] [PubMed] [Google Scholar]
- 48.Hill K, Model K, Ryan M T, Dietmeier K, Martin F, Wagner R, Pfanner N. Nature (London) 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- 49.Rapaport D, Neupert W, Lill R. J Biol Chem. 1997;272:18725–18731. doi: 10.1074/jbc.272.30.18725. [DOI] [PubMed] [Google Scholar]
- 50.Rapaport D, Mayer A, Neupert W, Lill R. J Biol Chem. 1998;273:8806–8813. doi: 10.1074/jbc.273.15.8806. [DOI] [PubMed] [Google Scholar]
- 51.Brix J, Rudiger S, Bukau B, Schneider-Mergener J, Pfanner N. J Biol Chem. 1999;274:16522–16530. doi: 10.1074/jbc.274.23.16522. [DOI] [PubMed] [Google Scholar]
- 52.Schleyer M, Neupert W. Cell. 1985;43:330–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- 53.Martin J, Mahlke K, Pfanner N. J Biol Chem. 1991;266:18051–18057. [PubMed] [Google Scholar]
- 54.Corpet F. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rost B, Sander C. Proteins. 1994;20:216–226. doi: 10.1002/prot.340200303. [DOI] [PubMed] [Google Scholar]