Abstract
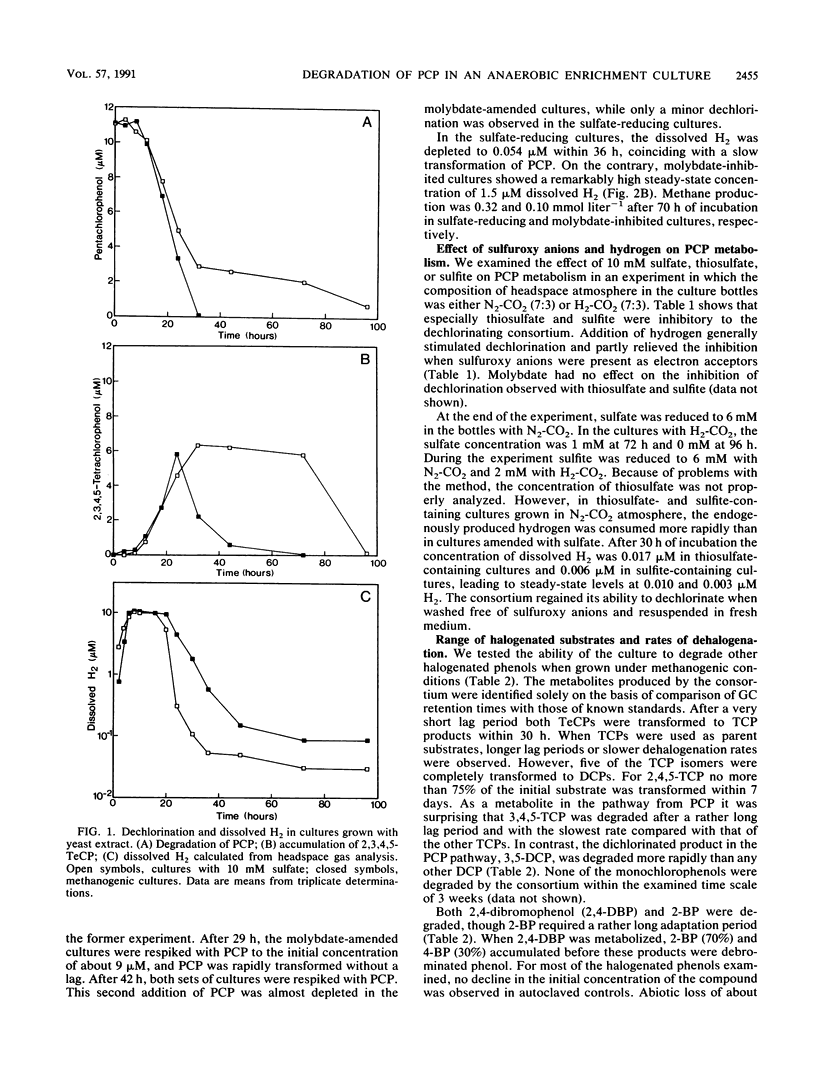
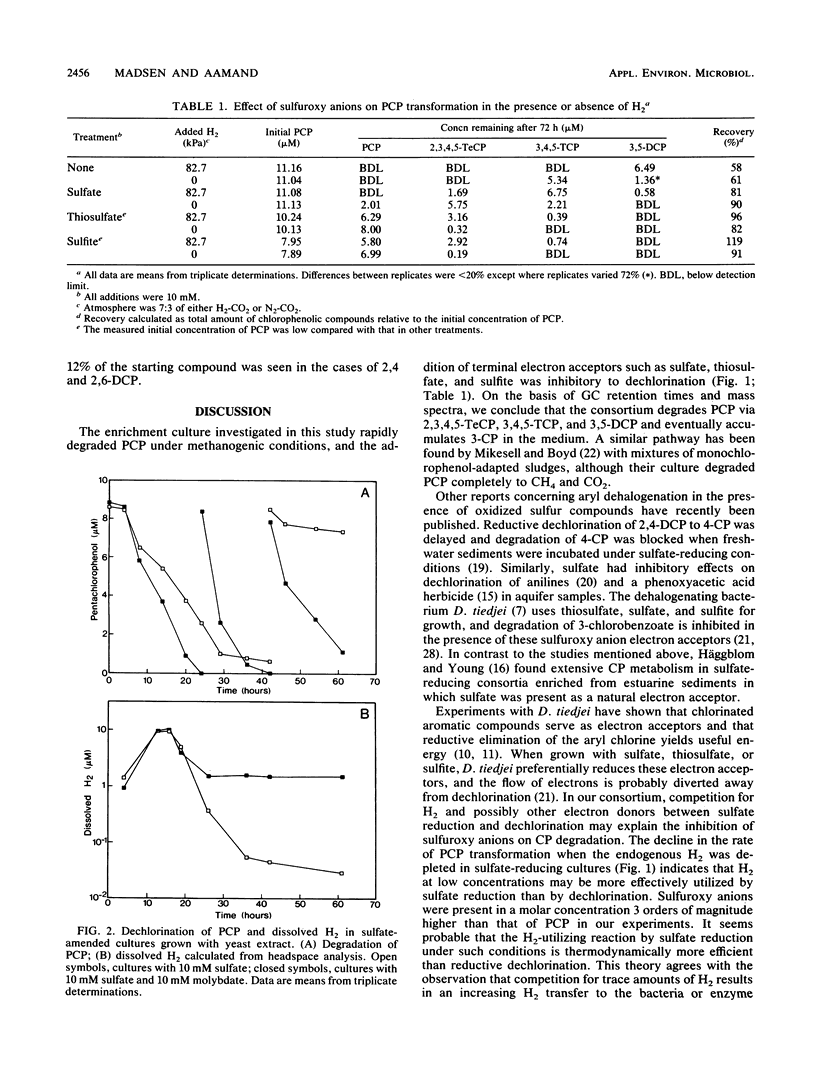
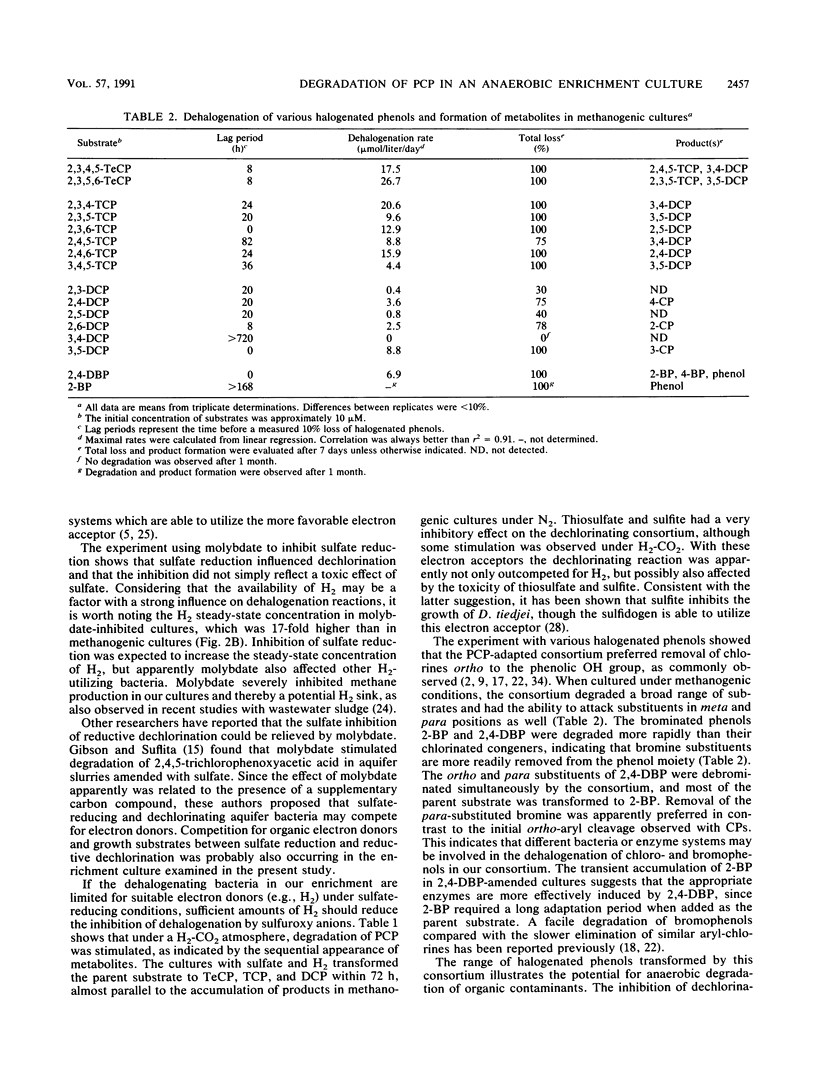
We studied the degradation of pentachlorophenol (PCP) under methanogenic and sulfate-reducing conditions with an anaerobic mixed culture derived from sewage sludge. The consortium degraded PCP via 2,3,4,5-tetrachlorophenol, 3,4,5-trichlorophenol, and 3,5-dichlorophenol and eventually accumulated 3-chlorophenol. Dechlorination of PCP and metabolites was inhibited in the presence of sulfate, thiosulfate, and sulfite. A decrease in the rate of PCP transformation was noted when the endogenous dissolved H2 was depleted below 0.11 μM in sulfate-reducing cultures. The effect on dechlorination observed with sulfate could be relieved by addition of molybdate, a competitive inhibitor of sulfate reduction. Addition of H2 reduced the inhibition observed with sulfuroxy anions. The inhibitory effect of sulfuroxy anions may be due to a competition for H2 between sulfate reduction and dechlorination. When cultured under methanogenic conditions, the consortium degraded several chlorinated and brominated phenols.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R., Berry D., Tiedje J. M. Anaerobic biodegradation of phenolic compounds in digested sludge. Appl Environ Microbiol. 1983 Jul;46(1):50–54. doi: 10.1128/aem.46.1.50-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Chapalamadugu S. Biodegradation of halogenated organic compounds. Microbiol Rev. 1991 Mar;55(1):59–79. doi: 10.1128/mr.55.1.59-79.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweerd K. A., Suflita J. M. Anaerobic Aryl Reductive Dehalogenation of Halobenzoates by Cell Extracts of "Desulfomonile tiedjei". Appl Environ Microbiol. 1990 Oct;56(10):2999–3005. doi: 10.1128/aem.56.10.2999-3005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153(3):264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogenic coculture. Arch Microbiol. 1987;149(2):102–105. doi: 10.1007/BF00425073. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Anaerobic Degradation of Chloroaromatic Compounds in Aquatic Sediments under a Variety of Enrichment Conditions. Appl Environ Microbiol. 1989 Jun;55(6):1466–1471. doi: 10.1128/aem.55.6.1466-1471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Characterization of anaerobic dechlorinating consortia derived from aquatic sediments. Appl Environ Microbiol. 1989 Jun;55(6):1472–1476. doi: 10.1128/aem.55.6.1472-1476.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Anaerobic biodegradation of 2,4,5-trichlorophenoxyacetic Acid in samples from a methanogenic aquifer: stimulation by short-chain organic acids and alcohols. Appl Environ Microbiol. 1990 Jun;56(6):1825–1832. doi: 10.1128/aem.56.6.1825-1832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Young L. Y. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990 Nov;56(11):3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Dehalogenation in marine sediments containing natural sources of halophenols. Appl Environ Microbiol. 1988 Dec;54(12):3079–3085. doi: 10.1128/aem.54.12.3079-3085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Zhang X. M., Wiegel J. Anaerobic dechlorination of 2,4-dichlorophenol in freshwater sediments in the presence of sulfate. Appl Environ Microbiol. 1989 Oct;55(10):2735–2737. doi: 10.1128/aem.55.10.2735-2737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. P., Townsend G. T., Suflita J. M. Effect of sulfate and organic carbon supplements on reductive dehalogenation of chloroanilines in anaerobic aquifer slurries. Appl Environ Microbiol. 1990 Sep;56(9):2630–2637. doi: 10.1128/aem.56.9.2630-2637.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkfield T. G., Tiedje J. M. Characterization of the requirements and substrates for reductive dehalogenation by strain DCB-1. J Ind Microbiol. 1990 Jan;5(1):9–15. doi: 10.1007/BF01569601. [DOI] [PubMed] [Google Scholar]
- Mikesell M. D., Boyd S. A. Complete reductive dechlorination and mineralization of pentachlorophenol by anaerobic microorganisms. Appl Environ Microbiol. 1986 Oct;52(4):861–865. doi: 10.1128/aem.52.4.861-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. O., Linkfield T. G., Tiedje J. M. Physiological characterization of strain DCB-1, a unique dehalogenating sulfidogenic bacterium. Appl Environ Microbiol. 1988 Dec;54(12):2938–2943. doi: 10.1128/aem.54.12.2938-2943.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976 Dec 10;194(4270):1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wiegel J. Sequential anaerobic degradation of 2,4-dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990 Apr;56(4):1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



