Abstract
The import of cytochrome b2 into mitochondria consists of two steps. The translocation of the first part of the presequence across the inner membrane is coupled with the translocation of the tightly folded heme-binding domain across the outer membrane and requires a membrane potential ΔΨ and the functions of mitochondrial Hsp70 (mHsp70) in the matrix. Once the heme-binding domain has passed the outer membrane, the translocation of the rest of the polypeptide chain across the outer membrane becomes independent of ΔΨ and mHsp70. Here we analyzed the late ΔΨ- and mHsp70-independent step in the transport of cytochrome b2 fusion proteins into the intermembrane space (IMS). The import of the cytochrome b2 fusion proteins containing two protein domains linked by a spacer segment into mitochondria was arrested at a stage at which one domain folded on each side of the outer membrane, along the pathway that is consistent with the stop-transfer model. The mature-size form of the translocation intermediate could move across the outer membrane in both directions, and the stabilization of the protein domain in the IMS promoted the forward translocation. On the other hand, the intermediate-size form of the translocation intermediate, which retains the anchorage to the inner membrane, was transported into the IMS independently of the stability of the protein domain in the IMS. These results suggest that two distinct mechanisms, the Brownian ratchet and the anchor diffusion mechanisms, can operate for the transmembrane movement of the mature-size form and the intermediate-size form, respectively, of cytochrome b2 species.
Newly synthesized proteins have to traverse several biological membranes when the sites of their synthesis and of their functions are separated by lipid bilayers. Recent studies have shown that polypeptide chains move across the membrane through a hydrophilic hetero-oligomeric transmembrane channel composed of integral membrane proteins (1–3). However, the question of what drives translocation of the protein through the channel from one side of the membrane to the other still remains a mystery.
Many mitochondrial proteins are synthesized in the cytosol, imported into mitochondria, and sorted to one of the four mitochondrial compartments, the outer membrane, the inner membrane, the intermembrane space (IMS), and the matrix, along many different pathways with the aid of the TOM and the TIM complexes (the translocator complexes in the outer and the inner membranes, respectively) (for reviews, see refs. 4–10). The different protein sorting pathways may use different mechanisms to translocate proteins across the mitochondrial membranes. The import of proteins into the mitochondrial matrix requires both a membrane potential (ΔΨ) across the inner membrane and ATP hydrolysis in the matrix. ΔΨ is essential for the entry of the N-terminal mitochondrial targeting signal of the precursor protein into the matrix. The energy of ATP hydrolysis is used by mitochondrial Hsp70 (mHsp70) for the unfolding of the rest of the precursor proteins and the subsequent translocation of the polypeptide chain across the inner membrane, although the precise mechanism of the action of this process is controversial.
On the other hand, the driving force for the transmembrane movement of the proteins into the IMS is unclear. The precursor of cytochrome b2 possesses an N-terminal 80-residue bipartite presequence and is transported to the IMS. The first part of the presequence directs the protein to mitochondria and is proteolytically cleaved off by the mitochondrial processing peptidase in the matrix, generating an intermediate-size form, and the second part of the presequence mediates the transfer of the protein to the IMS and is removed by the inner-membrane protease I (Imp1p) on the IMS side of the inner membrane to yield a mature-size form. The mature part of cytochrome b2 contains a heme-binding domain (HBD) in the N-terminal region (residues 81–180), which can fold independently of the rest of the molecule (11, 12). The import pathway for the cytochrome b2 precursor to the IMS has been a matter of debate for many years (4–6). In the “conservative sorting” model, the cytochrome b2 precursor is first translocated across both the outer and the inner membranes to reach the matrix and then reexported to the IMS. In the “stop-transfer” model, the second part of the presequence arrests the translocation across the inner membrane, so that the mature part crosses only the outer membrane to reach the IMS. In both models, the translocation of the first part of the presequence across the inner membrane is coupled with the translocation of the HBD across the outer membrane and is driven by ΔΨ and ATP-dependent mHsp70 (11, 13, 14). However, once the tightly folded HBD has passed the outer membrane, the subsequent translocation of the rest of the polypeptide chain across the outer membrane becomes independent of ΔΨ and mHsp70 (11, 13, 14). The outer membrane lacks a transmembrane potential, and no ATP-dependent chaperone has been found in the IMS. Then, what drives the translocation of cytochrome b2 across the outer membrane?
In the present study, we analyzed the late ΔΨ- and mHsp70-independent step in the transport of cytochrome b2 fusion proteins into the IMS. The import of cytochrome b2 fusion proteins containing two protein domains linked by a spacer segment into the mitochondria was arrested at a stage at which the N-terminal and the C-terminal domains folded on the trans side and the cis side, respectively, of the outer membrane. The mature-size form of the translocation intermediate, which lost the anchorage to the inner membrane, was found to move across the outer membrane in both directions, and the stabilization of the protein domain on the trans side enhanced the rate of the forward translocation to the trans side. On the other hand, the intermediate-size form of the translocation intermediate, which retained the anchorage to the inner membrane, was transported to the trans side of the membrane independently of the stability of the protein domain on the trans side. These results suggest that two distinct mechanisms, the Brownian ratchet and the anchor diffusion mechanisms, can operate for the transmembrane movement of the mature-size form and the intermediate-size form, respectively, of the cytochrome b2 species.
Materials and Methods
Plasmids.
The genes for pb2(220)dc1-mouse dihydrofolate reductase (DHFR) and pb2(220)dc2-DHFR (Fig. 1) were derived from that for pb2(220)WT-mouse dihydrofolate reductase (DHFR) (15). In brief, codons for His123 and His146 of the gene for pb2(220)WT-DHFR were replaced by those for Leu123 and Leu146 to yield the gene for pb2(220)dc1-DHFR, and codons for Phe142 and Glu143 by those for Pro142 and Pro143 to yield the gene for pb2(220)dc2-DHFR by oligonucleotides-directed mutagenesis (16). Codons for Asn80 and Glu81 of pb2(220)WT-DHFR were replaced by those for Ala80 and Ala81 to yield the gene for pb2AA(220)WT-DHFR. The genes for pb2AA(220)dc1-DHFR and pb2AA(220)dc2-DHFR were constructed by replacing the DNA segments for the first 85 amino acid residues of pb2(220)dc1-DHFR and pb2(220)dc2-DHFR, respectively, with that taken from the pb2AA(220)WT-DHFR gene. For constructing the gene for pb2(260)WT-HBD, the SpeI site and the TAA stop codon were introduced at the 5′-end and the 3′-end of the DNA fragment, respectively, for residues 81–189 of cytochrome b2 by PCR. The amplified fragment was inserted into the cytochrome b2 gene at the SpeI site, which had been introduced at the codons for residues 259 and 260 with the concomitant replacement of Ile259 by Thr259. The genes for pb2(260)dc1-HBD and pb2(260)dc2-HBD were constructed by replacing the DNA segment for the first 200 amino acid residues of pb2(260)WT-HBD with those taken from the genes for pb2(220)dc1-DHFR and pb2(220)dc2-DHFR, respectively.
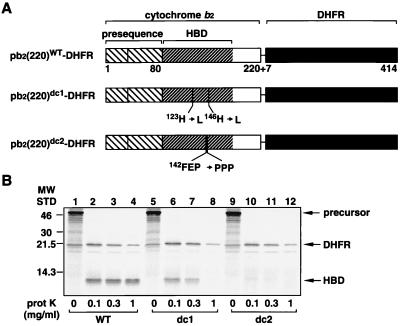
Figure 1.
(A) pb2(220)-DHFR fusion proteins. (B) A proteinase K digestion of the pb2(220)-DHFR fusion proteins. pb2(220)WT-DHFR (WT), pb2(220)dc1-DHFR (dc1), and pb2(220)dc2-DHFR (dc2) synthesized in vitro were diluted 100-fold with 250 mM sucrose, 10 mM Mops⋅KOH (pH 7.2), 80 mM KCl, 2 mM cold methionine, and 4 μM hemin and were treated with various concentrations of proteinase K (prot K) for 10 min at 25°C. The reactions were stopped by the addition of 1 mM phenylmethylsulfonyl fluoride, and proteins were precipitated by trichloroacetic acid and were analyzed by SDS/PAGE and radioimaging.
Import of the Cytochrome b2 Fusion Proteins into Mitochondria.
The fusion proteins were synthesized in a cell-free translation system with reticulocyte lysate in the presence of [35S]methionine and 20 μM hemin. Mitochondria were isolated from the yeast strain D273-10B (17). The radiolabeled fusion proteins were incubated with mitochondria in import buffer (250 mM sucrose/10 mM Mops⋅KOH, pH 7.2/80 mM KCl/5 mM MgCl2/2.5 mM KPi/5 mM DTT/2 mM methionine/1% BSA/2 mM ATP/2 mM NADH) at 25°C. The import reaction was stopped by adding valinomycin to 10 μg/ml. The mitochondria were reisolated by centrifugation, and proteins were analyzed by SDS/PAGE and radioimaging with a Storm 860 image analyzer (Molecular Dynamics).
Methotrexate Arrest of the pb2(220)-DHFR Fusion Proteins and Chase Reactions.
The translation products (5%) containing radiolabeled pb2(220)-DHFR fusion proteins were preincubated with 0.5 μM methotrexate (MTX)/1 mM NADPH in import buffer for 15 min on ice and were subsequently incubated with isolated yeast mitochondria for 15 min at 25°C. The MTX block of the import was confirmed by protease treatment of the mitochondria. For chase reactions, the mitochondria without protease treatment were reisolated by centrifugation, were washed once with import buffer containing 0.5 μM recombinant DHFR, were resuspended in import buffer without BSA and ATP but containing 3 μM hemin, and were incubated at 25°C. The import of the arrested fusion proteins was followed by monitoring the amounts of protease-protected proteins. The suborganellar localization of the imported proteins was assessed by the methods described previously (18).
Kinetic Arrest of the pb2(260)-HBD Fusion Proteins and Chase Reactions.
The radiolabeled pb2(260)-HBD fusion proteins were incubated with isolated yeast mitochondria in import buffer for 10 min at 25°C. The kinetic block of the import was confirmed by protease treatment of the mitochondria. For chase reactions, the mitochondria without protease treatment were reisolated by centrifugation, were resuspended in import buffer without BSA (and hemin), and were incubated at 25°C. The mitochondria were divided into halves and were incubated with or without 100 μg/ml proteinase K for 30 min on ice. The import of the arrested fusion proteins and its suborganellar localization was followed as described above.
Results and Discussion
Cytochrome b2 Fusion Proteins Are Transported into the IMS Along the Pathway Consistent with the Stop-Transfer Model.
We assessed the sorting pathway for cytochrome b2 by using derivatives of IMS-targeting pb2(220)WT-DHFR, a fusion protein between the N-terminal 220 residues of the cytochrome b2 precursor and DHFR (Fig. 1A). Residues 1–80 and residues 81–180 of the cytochrome b2 part correspond to the presequence and the HBD, respectively. It is known that the efficient processing of the cytochrome b2 presequence by Imp1p requires the presence of a correctly folded HBD that is stabilized by the bound heme group (11, 12). We introduced mutations in the HBD to destabilize its folded conformation (Fig. 1A). In pb2(220)dc1-DHFR, His123 and His146, ligands for the heme Fe(II), are replaced by leucine residues so that the heme Fe(II) cannot coordinate with the HBD. In pb2(220)dc2-DHFR, an α-helical structure is destabilized by the replacement of the segment Phe142Glu143Pro144 by Pro142Pro143Pro144. The fusion proteins were synthesized in a cell-free translation system with rabbit reticulocyte lysate in the presence of [35S]methionine and were subjected to a digestion with proteinase K. As expected, the proteinase K treatment showed that pb2(220)WT-DHFR yielded a protease-resistant HBD core fragment whereas pb2(220)dc1-DHFR and pb2(220)dc2-DHFR did not (Fig. 1B). Thus, the HBD structure is significantly destabilized in pb2(220)dc1-DHFR and pb2(220)dc2-DHFR as compared with pb2(220)WT-DHFR.
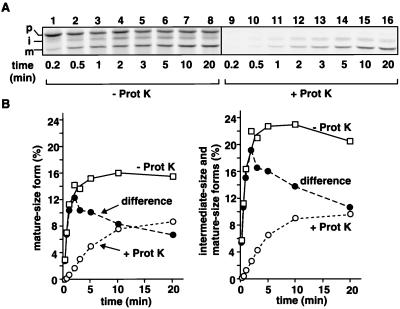
When incubated with isolated yeast mitochondria, the radiolabeled fusion protein, pb2(220)WT-DHFR, was imported into mitochondria (Fig. 2) and correctly localized to the IMS (not shown). A part of the mature-size form, which received the second Imp1p processing of the presequence, still remained outside the mitochondria because it was protease-sensitive (Fig. 2B). The protease-sensitive mature-size form increased in early time points until ≈3–5 min of incubation, but then its level declined upon further incubation. The fusion proteins with destabilized HBDs, pb2(220)dc1-DHFR and pb2(220)dc2-DHFR, also were imported into the IMS, although the Imp1p processing was significantly retarded as compared with that of pb2(220)WT-DHFR (data not shown).
Figure 2.
In vitro import of pb2(220)WT-DHFR into mitochondria. (A) Radiolabeled pb2(220)WT-DHFR was incubated with isolated yeast mitochondria at 25°C for indicated times. The mitochondria were treated with (+ Prot K, lanes 1–8) or without (− Prot K, lanes 9–16) 100 μg/ml proteinase K for 30 min on ice. (B) Amounts of the total mature-size form (A, lanes 1–8, open squares), those of the protease-protected mature-size form (A, lanes 9–16, open circles), which received the Imp1p processing and was already localized in the IMS, and those of the protease-sensitive mature-size form (filled circles), which received the Imp1p processing but still remained outside the mitochondria, are plotted against incubation times in the left panel. Amounts of the total mature-size and intermediate-size forms (A, lanes 1–8, open squares), those of the protease-protected mature-size and intermediate-size forms (A, lanes 9–16, open circles), which were already localized in the IMS, and those of the protease-sensitive mature-size and intermediate-size forms (filled circles), which still remained outside of the mitochondria, are plotted against incubation times in the right panel. The amount of the fusion proteins added to the reaction is set to 100%. p, precursor; i, intermediate-size form; m, mature-size form.
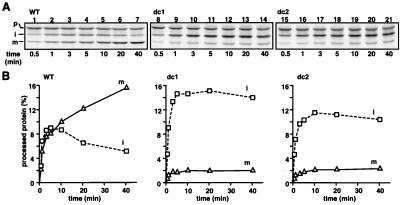
Because the translocation across the mitochondrial membranes requires unfolding of the passenger protein, the stabilization of the tertiary structure of the DHFR domain of cytochrome b2 fusion proteins by a ligand MTX blocks the translocation of the DHFR part across the outer membrane, resulting in the formation of translocation intermediates (19, 20). In these intermediates, the DHFR moiety remains outside the outer membrane whereas the N terminus reaches the IMS. According to the stop-transfer model, the intermediate spans only one membrane whereas, according to the conservative sorting model, it spans three membranes looping through the matrix (19, 20). In the former case, the HBD is able to fold in the IMS whereas, in the latter case, the HBD cannot fold because the major part of the HBD must form the loop. We found that the Imp1p processing of the translocation intermediates of pb2(220)dc1-DHFR and pb2(220)dc2-DHFR was significantly retarded as compared with that of the wild-type fusion protein (Fig. 3). Although the wild-type fusion protein generated a mature-size form efficiently within 10 min of incubation, the majority of the fusion proteins with the destabilized HBD was still present as intermediate-size forms even after 40 min of incubation. The more efficient Imp1p processing for the wild-type fusion protein than that for the HBD-destabilized mutants suggests that the HBD in the translocation intermediate of pb2(220)WT-DHFR can fold tightly and bind to heme in the IMS. This experiment was inspired by the previous study (19) in which the presence of the protease-resistant HBD of MTX-arrested pb2(219)WT-DHFR in the IMS was confirmed by rupturing the outer membrane followed by a protease digestion. Although this earlier approach could not rule out the possibility that the HBD may have folded after cleavage by Imp1p, the present approach avoids such a problem by using Imp1p cleavage itself as an indicator of the HBD folding. These results are thus consistent with the stop-transfer model but not with the conservative-sorting model, at least under the present experimental conditions.
Figure 3.
The tight folding of the HBD promotes the Imp1p processing of the translocation intermediates of the pb2(220)-DHFR fusion proteins. (A) Radiolabeled pb2(220)WT-DHFR (WT), pb2(220)dc1-DHFR (dc1), and pb2(220)dc2-DHFR (dc2) were preincubated with 1 μM MTX/1 mM NADPH in import buffer for 15 min on ice and subsequently were incubated with yeast mitochondria at 25°C for indicated times. (B) Amounts of the intermediate-size forms and the mature-size forms. The amounts of the fusion proteins added to each reaction are set to 100%. p, precursor; i, intermediate-size form; m, mature-size form.
The MTX-Arrested Mature-size pb2(220)-DHFR Fusion Proteins Can Be Efficiently Chased into the IMS After Removal of MTX.
In the intermediate of the fusion proteins complexed with MTX, the HBD has already passed the outer membrane whereas the following DHFR domain bound to MTX remained outside the mitochondria. The chase of the intermediate by the removal of MTX therefore corresponds to the late step of the transport of the fusion proteins into the IMS, which is independent of ΔΨ and mHsp70 (19, 20). The stop-transfer model predicts that, in the translocation intermediate complexed with MTX, the N terminus of the mature-size form is free in the IMS and is not associated with the inner membrane whereas the N terminus of the intermediate-size form is anchored to the inner membrane (19, 20). The blue-native gel electrophoresis analyses of the digitonin-lysed mitochondria (21, 22) showed that the translocation intermediate of the 38-kDa mature-size form is in an ≈500-kDa complex containing Tom40, which is in an ≈440-kDa complex in the absence of the translocation intermediate (not shown). This is consistent with the prediction that the translocation intermediate of the mature-size form is associated only with the TOM complex. On the other hand, the translocation intermediate of the 43-kDa intermediate-size form is in an ≈600-kDa complex containing Tom40 and probably unidentified TIM proteins but without Tim23 (not shown). This result is consistent with the previous suggestion that the translocation intermediate of the intermediate-size form is already unloaded from the TIM complex consisting of Tim23, Tim17, and Tim44 (14, 15).
We tested whether the mature-size forms of the MTX-arrested fusion proteins would complete their translocation into the IMS after the removal of MTX. The fusion proteins, pb2(220)dc1-DHFR, pb2(220)dc2-DHFR, and pb2(220)WT-DHFR, were incubated with mitochondria in the presence of MTX to generate translocation intermediates (first incubation). The mitochondria then were reisolated, were suspended in fresh import buffer without MTX, and were subjected to further incubation at 25°C (second incubation). The Imp1p-processed mature form of the accumulated wild-type fusion protein was efficiently chased into the IMS when the MTX-arrest was relieved by the removal of MTX (Fig. 4 A, WT and B, in/WT). Here the correct localization of the chased species in the IMS was confirmed by the protease digestion of the mitochondria and the mitoplasts (not shown). Thirty-eight percent of the accumulated fusion protein was chased into the IMS after 20 min of the second incubation. This chase is highly efficient because the chased fraction (38%) corresponds to 12% of the input, which is comparable to the amount of the mature form that was directly imported in the absence of MTX (Fig. 2B). Previously, the MTX-arrested mature-size cytochrome b2 species were predicted to be a dead-end intermediate in the stop-transfer model (19), and the successful chase of the intermediate into the IMS was interpreted as strong evidence for the conservative-sorting model (20). Then, how can we reconcile the apparently divergent observations, the tightly folded HBD of MTX-arrested pb2(220)WT-DHFR without looping through the inner membrane and the successful chase of the MTX-arrested mature-size forms of the pb2(220) -DHFR fusion proteins into the IMS?
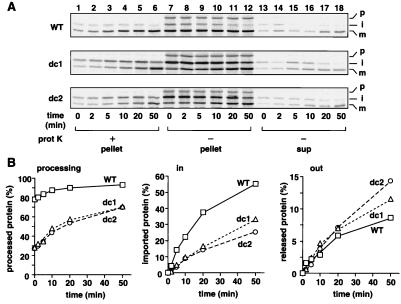
Figure 4.
The tightly folded HBD in the IMS promotes the chase of the mature-size forms of the MTX-arrested pb2(220)-DHFR fusion proteins into the IMS when MTX is removed. (A) Radiolabeled pb2(220)WT-DHFR (WT), pb2(220)dc1-DHFR (dc1), and pb2(220)dc2-DHFR (dc2) were incubated with isolated yeast mitochondria at 25°C in the presence of 0.5 μM MTX. The mitochondria were reisolated by centrifugation, were washed once, and were incubated at 25°C for indicated times. The mitochondria were divided into halves and were incubated with (+ prot K, lanes 1–6) or without (− prot K, lanes 7–18) 100 μg/ml proteinase K for 30 min on ice. The mitochondria without proteinase K treatment were reisolated by centrifugation, and proteins recovered with the mitochondria (pellet) and those in the supernatant (sup) were analyzed by SDS/PAGE and radioimaging. p, precursor; i, intermediate-size form; m, mature-size form. (B) Amounts of the forms processed by Imp1p [the mature-size forms in “pellet” (A, lanes 7–12) and “sup” (A, lanes 13–18)] are plotted against incubation times in the left panel. Amounts of the imported proteins [the protease-protected mature-size forms (A, lanes 1–6)] are plotted against incubation times in the center panel; the amounts at time 0 are set to 0%. Amounts of the retrotranslocated proteins [the mature-size forms in “sup” (A, lanes 13–18)] are plotted against incubation times in the right panel; the amounts at time 0 are set to 0%. The amounts of the MTX-arrested fusion proteins associated with mitochondria after the first incubation are set to 100%. Squares, wild type (WT); triangles, dc1; circles, dc2.
We noticed that, when the mitochondria were recovered after the second incubation, a portion of the mature form of the accumulated wild-type fusion protein was translocated back into the supernatant (Fig. 4 A, WT and B, out/WT). The reversibility of the translocation of the mature form of the arrested fusion protein may well reflect the fact that the TOM channel in the outer membrane represents a passive import channel, which interacts only weakly with the translocating polypeptide chains (23). We further found that the stability of the HBD in the IMS, which had already crossed the outer membrane, significantly affects the efficiencies of the forward translocation vs. the retrograde translocation of the arrested fusion proteins. The HBD-destabilized mutants of the pb2(220)-DHFR fusion proteins were imported into the mitochondria less efficiently than pb2(220)WT-DHFR (Fig. 4 A and B, in/WT, dc1 and dc2). On the other hand, the HBD-destabilized mutants were released outside of the mitochondria slightly more efficiently than pb2(220)WT-DHFR (Fig. 4 A and B, out/WT, dc1 and dc2).
The Brownian-Ratchet Mechanism Can Drive Protein Translocation of Mature-Size Cytochrome b2 Fusion Proteins Across the Outer Membrane.
Next, we prepared the pb2(260)-HBD fusion proteins, pb2(260)WT-HBD, pb2(260)dc1-HBD, and pb2(260)dc2-HBD, in which the first HBD (HBDWT, HBDdc1, and HBD dc2, respectively) is followed by a 80-residue spacer segment fused to the second wild-type HBD (Fig. 5A). These fusion proteins were incubated with energized mitochondria, and the mitochondria were reisolated by centrifugation. Treatment of the reisolated mitochondria with externally added protease followed by centrifugation yielded a protease-resistant C-terminal fragment arising from the second HBD in the supernatant and the N-terminal fragment consisting of the first HBD and a spacer segment in the mitochondrial fraction (not shown). This indicates that the first HBD was efficiently translocated across the outer membrane (and likely rebound to heme) whereas the second HBD (complexed with heme) remained outside the mitochondria. This membrane topology of the partially translocated fusion proteins, in which the two HBDs were on both sides of the outer membrane, allowed us to further characterize the protein translocation across the outer membrane.
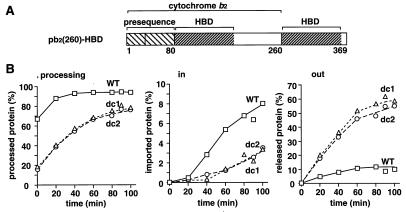
Figure 5.
Destabilization of the HBD in the IMS promotes the retrograde translocation of the kinetically arrested translocation intermediates of the pb2(260)-HBD fusion proteins. (A) pb2(260)-HBD fusion proteins. (B) Radiolabeled pb2(260)WT-HBD (WT), pb2(260)dc1-HBD (dc1), and pb2(260)dc2-HBD (dc2) were incubated with isolated yeast mitochondria in import buffer for 10 min at 25°C. The mitochondria were reisolated by centrifugation and were incubated at 25°C for indicated times. The mitochondria were divided into halves and were incubated with or without 100 μg/ml proteinase K for 30 min on ice. The mitochondria without proteinase K treatment were reisolated by centrifugation, and proteins recovered with the mitochondria (pellet) and those in the supernatant (sup) were analyzed by SDS/PAGE and radioimaging. Amounts of the forms processed by Imp1p (the mature-size forms in “pellet” and “sup”) are plotted against incubation times in the left panel. Amounts of the imported proteins (the protease-protected mature-size forms) are plotted against incubation times in the center panel; the amounts at time 0 are set to 0%. Amounts of the retrotranslocated proteins (the mature-size forms in “sup”) are plotted against incubation times in the right panel; the amounts at time 0 are set to 0%. The amounts of the kinetically arrested fusion proteins associated with mitochondria after the first incubation are set to 100%. Squares, wild type (WT); triangles, dc1; circles, dc2.
The reisolated mitochondria containing the partially translocated pb2(260)-HBD fusion proteins were suspended in fresh import buffer and were subjected to incubation at 25°C. The mature-size form of the accumulated wild-type fusion protein was slowly chased into the IMS (Fig. 5B, in/WT). About 9% of the arrested pb2(260)WT -HBD was chased to the trans side of the outer membrane or the IMS after 100 min of the incubation. At the same time, a similar amount of the fusion protein was translocated back to the cis side of the membrane (Fig. 5B, out/WT). The destabilization of the first HBD on the trans side of the membrane promoted the retrotranslocation of the pb2(260)-HBD fusion proteins to the cis side; pb2(260)dc1-HBD and pb2(260)dc2-HBD were released outside of the mitochondria more efficiently than they were imported into the IMS (Fig. 5B, in/dc1 and dc2, out/dc1 and dc2).
These results with the pb2(220)-DHFR and pb2(260)-HBD fusion proteins can be interpreted in the framework of the simplest version of the Brownian ratchet model for the protein translocation (24, 25). The arrested fusion proteins have folded domains on both sides of the outer membrane, the DHFR/HBD domain on the cytosolic cis side and the HBD on the IMS trans side. The breathing unfolding of the folded cis-side domain would allow the forward movement of an extended segment in the import channel of the TOM complex (26). The breathing unfolding of the folded trans-side HBD would conversely allow the backward movement of an extended segment of the fusion protein into the import channel. Then Brownian thermal motions would cause the unfolded segment to diffuse in both directions through the import channel. The forward movement toward the trans side would be favorable for the (re)folding of the trans-side HBD. The backward movement toward the cis side would be favored by the (re)folding of the cis-side domain. The accumulation of these fluctuating movements would result in the net displacement of the polypeptide segment in the import channel. This displacement of the polypeptide segment would lead to the irreversible release of the fusion protein to the trans side or to the cis side of the membrane. When the folded structure of the HBD on the trans side is more destabilized, the HBD cannot function as an efficient ratchet for the forward Brownian movement. This will suppress the net displacement of the fusion proteins in the inward direction as observed for both the pb2(220)-DHFR and pb2(260)-HBD fusion proteins (Fig. 4 and 5). When the forward translocation is not so efficient, the destabilization of the trans-side HBD conversely promotes the net displacement of the fusion protein in the outward direction, resulting in the faster release of the fusion protein to the cis side of the membrane as observed in Fig. 5.
Translocation Intermediates of Intermediate-Size pb2AA(220)-DHFR Fusion Proteins Can Be Transported into the IMS by the Anchor Diffusion Mechanism.
Finally, we compared the kinetics of the translocation of the mature-size form with those of the intermediate-size form, which still retains the anchorage of their presequence to the inner membrane but is disengaged from the mHsp70 functions (14, 15). For this purpose, we used pb2AA(220)-DHFR fusion proteins, which do not virtually receive the second processing of the presequence by Imp1p because the processing site for Imp1p is mutated (Fig. 6A). In contrast to the pb2(220)-DHFR fusion proteins, the translocation of the pb2AA(220)-DHFR fusion proteins into the IMS did not depend on the stability of the first HBD. The MTX-arrested fusion proteins pb2AA(220)WT-DHFR, pb2AA(220)dc1-DHFR, and pb2AA(220)dc2-DHFR were transported into the IMS with nearly the same kinetics when MTX was removed (Fig. 6B). Essentially the same results were obtained with the kinetically arrested pb2AA(260)-HBD fusion proteins, although translocation efficiency was low (data not shown). These results suggest that the intermediate-size forms of the pb2AA(220)-DHFR fusion proteins move across the outer membrane by a mechanism different from the Brownian ratchet model. Because the pb2AA(220)-DHFR fusion proteins are anchored to the inner membrane by their sorting signals, the lateral diffusion of the anchorage of the sorting signal in the inner membrane may well drive the translocation of the rest of the polypeptide chain across the outer membrane (“anchor diffusion model”) (27). It may be worth noting that, because the Imp1p processing of the HBD-destabilized mutants is slow (Fig. 4A, dc1 and dc2; data not shown), the forward translocation, but not the backward translocation, of the HBD-destabilized mutants, but not of the wild-type fusion protein, into the IMS (Fig. 4B, in/dc1 and dc2, and Fig. 5B, in/dc1 and dc2) may be partly driven by the anchor diffusion mechanism.
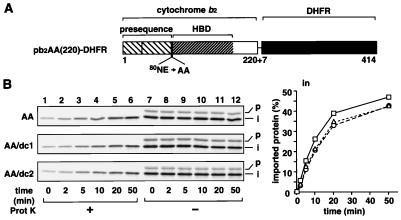
Figure 6.
The stability of the HBD in the IMS does not affect the chase of the intermediate-size forms of the MTx-arrested pb2(220)-DHFR fusion proteins into the IMS when MTX is removed. (A) pb2AA(220)-DHFR fusion proteins. (B) Generation of the translocation intermediates of radiolabeled pb2AA(220)WT-DHFR, pb2AA(220)dc1-DHFR, and pb2AA(220)dc2-DHFR at the outer membrane and the subsequent chase reactions were performed as described for Fig. 4. Amounts of the imported proteins (the protease-protected intermediate-size forms) are plotted against incubation times. Squares, AA; triangles, AA/dc1; circles, AA/dc2.
Biological Significance of the Transmembrane Movements Driven by the Anchor Diffusion and the Brownian Ratchet.
The results obtained here have demonstrated that the two distinct mechanisms can operate in the protein translocation across the outer membrane that is uncoupled from the translocation across the inner membrane mediated by mHsp70 in the matrix. The anchor diffusion mechanism likely drives the protein translocation across the outer membrane when the protein retains the anchorage of its presequence to the inner membrane. In this case, the folding of the translocating polypeptide chain on the trans side of the membrane does not affect the rate of the translocation. Inner-membrane proteins including d-lactate dehydrogenase (28) that are N-terminally anchored to the inner membrane and face to the IMS may well use this mechanism to move across the outer membrane.
The Brownian ratchet mechanism drives the protein translocation across the outer membrane when the anchorage of the presequence to the inner membrane is lost. The tight folding of the translocating polypeptide chain on the trans side of the membrane can ratchet the Brownian thermal motion and drive the translocation of the polypeptide chain through the import channel across the membrane. In the case of the import of cytochrome b2 species including authentic cytochrome b2 into mitochondria, because the second cleavage of the presequence by Imp1p occurs partly before complete translocation across the outer membrane (Fig. 2; ref. 18), not only the anchor diffusion mechanism but also the Brownian ratchet mechanism appear to operate for achieving the efficient protein translocation across the outer membrane along the stop-transfer pathway.
The rate and direction of the translocation across the membrane by the folding-driven Brownian ratchet depend on the stability of the folded protein domains on the cis and the trans sides of the membrane. The DHFR domain is less stable than the heme-bound HBD in the presence or absence of hemin when monitored by protease digestion (Fig. 1B; data not shown), and the translocation intermediate of pb2(220)WT-DHFR was more efficiently chased into the IMS than that of pb2(260)WT -HBD (Figs. 4 and 5). This indicates that the overall stability assessed by protease digestion, which reflects the global unfolding rates (29), of the cis-side domains appears to correlate with the rate of the transmembrane movement driven by the Brownian ratchet.
To speed protein translocation across the membranes in a unidirectional manner, eukaryotic cells may well have developed a mechanism of more efficient ratcheting working only on the trans side of the membranes at multiple sites along the polypeptide chain. ATP-dependent Hsp70 molecular chaperones recruited at the exit of the import channel may function as one of such ratchets (30, 31). However, when DHFR and HBD were fused to the mitochondrial matrix-targeting signals as passenger domains, the more stable HBD was even more efficiently imported into the matrix than DHFR (26). This suggests that translocation across the mitochondrial inner membrane mediated by mHsp70 cannot be explained by a simple Brownian ratchet mechanism and raises the possibility that other mechanisms, including active pulling by the Hsp70 translocation motor (32), may operate for this process.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan and a grant for the “Biodesign Research Program” from the Institute of Physical and Chemical Research (RIKEN). M.E. is a Research Fellow of the Japan Society for the Promotion of Science.
Abbreviations
- IMS
intermembrane space
- mHsp70
mitochondrial Hsp70
- HBD
heme-binding domain
- Imp1p
inner-membrane protease I
- MTX
methotrexate, DHFR, mouse dihydrofolate reductase
References
- 1.Hanein D, Matlack K E S, Jungnickel B, Plath K, Kalies K-U, Miller K R, Rapoport T A, Akey C W. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- 2.Künkele K-P, Heins S, Dembowski M, Nargang F E, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. Cell. 1998;93:1009–1019. doi: 10.1016/s0092-8674(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 3.Meyer T H, Ménétret J-F, Breitling R, Miller K R, Akey C W, Rapoport T A. J Mol Biol. 1999;285:1789–1800. doi: 10.1006/jmbi.1998.2413. [DOI] [PubMed] [Google Scholar]
- 4.Hartl F-U, Neupert W. Science. 1990;247:930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- 5.Glick B S, Beasley E M, Schatz G. Trends Biochem Sci. 1992;17:453–459. doi: 10.1016/0968-0004(92)90487-t. [DOI] [PubMed] [Google Scholar]
- 6.Stuart R A, Neupert W. Trends Biochem Sci. 1996;21:261–267. [PubMed] [Google Scholar]
- 7.Schatz G, Dobberstein B. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 8.Neupert W. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 9.Pfanner N, Craig E A, Hönlinger A. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Endo T. In: Molecular Chaperones in the Life Cycle of Proteins. Fink A L, Goto Y, editors. New York: Dekker; 1997. pp. 435–466. [Google Scholar]
- 11.Glick B S, Wachter C, Reid G A, Schatz G. Protein Sci. 1993;2:1901–1917. doi: 10.1002/pro.5560021112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart R A, Gruhler A, van der Klei I, Guiard B, Koll H, Neupert W. Eur J Biochem. 1994;220:9–18. doi: 10.1111/j.1432-1033.1994.tb18593.x. [DOI] [PubMed] [Google Scholar]
- 13.Gambill B D, Voos W, Kang P J, Miao B, Langer T, Craig E A, Pfanner N. J Cell Biol. 1993;123:109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gärtner F, Bömer U, Guiard B, Pfanner N. EMBO J. 1995;14:6043–6057. doi: 10.1002/j.1460-2075.1995.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanamori T, Nishikawa S, Shin I, Schultz P, Endo T. Proc Natl Acad Sci USA. 1997;94:485–490. doi: 10.1073/pnas.94.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 17.Daum G, Böhni P C, Schatz G. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- 18.Glick B S, Brandt A, Cunningham K, Müller S, Halleberg R L, Schatz G. Cell. 1992;69:809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- 19.Rospert S, Müller S, Schatz G, Glick B S. J Biol Chem. 1994;269:17279–17288. [PubMed] [Google Scholar]
- 20.Gruhler A, Ono H, Guiard B, Neupert W, Stuart R A. EMBO J. 1995;14:1349–1359. doi: 10.1002/j.1460-2075.1995.tb07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker P J T, Martin F, Maarse A C, Bömer U, Müller H, Guiard B, Meijer M, Rassow J, Pfanner N. EMBO J. 1997;16:5408–5419. doi: 10.1093/emboj/16.17.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schägger H, von Jagow G. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 23.Ungermann C, Neupert W, Cyr D M. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- 24.Neupert W, Hartl F-U, Craig E A, Pfanner N. Cell. 1990;63:447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- 25.Simon S M, Peskin C S, Oster G F. Proc Natl Acad Sci USA. 1992;89:3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaume B, Klaus C, Ungermann C, Guiard B, Neupert W, Brunner M. EMBO J. 1998;17:6497–6507. doi: 10.1093/emboj/17.22.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glick B, Wachter C, Schatz G. Trends Cell Biol. 1991;1:99–103. doi: 10.1016/0962-8924(91)90037-a. [DOI] [PubMed] [Google Scholar]
- 28.Rojo E E, Guiard B, Neupert W, Stuart R A. J Biol Chem. 1998;273:8040–8047. doi: 10.1074/jbc.273.14.8040. [DOI] [PubMed] [Google Scholar]
- 29.Imoto T, Yamada H, Ueda T. J Mol Biol. 1986;190:647–649. doi: 10.1016/0022-2836(86)90250-0. [DOI] [PubMed] [Google Scholar]
- 30.Schneider H-C, Berthold J, Bauer M F, Dietmeier K, Guiard B, Brunner M, Neupert W. Nature (London) 1994;371:768–774. doi: 10.1038/371768a0. [DOI] [PubMed] [Google Scholar]
- 31.Brodsky J L, Schekman R. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glick B S. Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]