Abstract
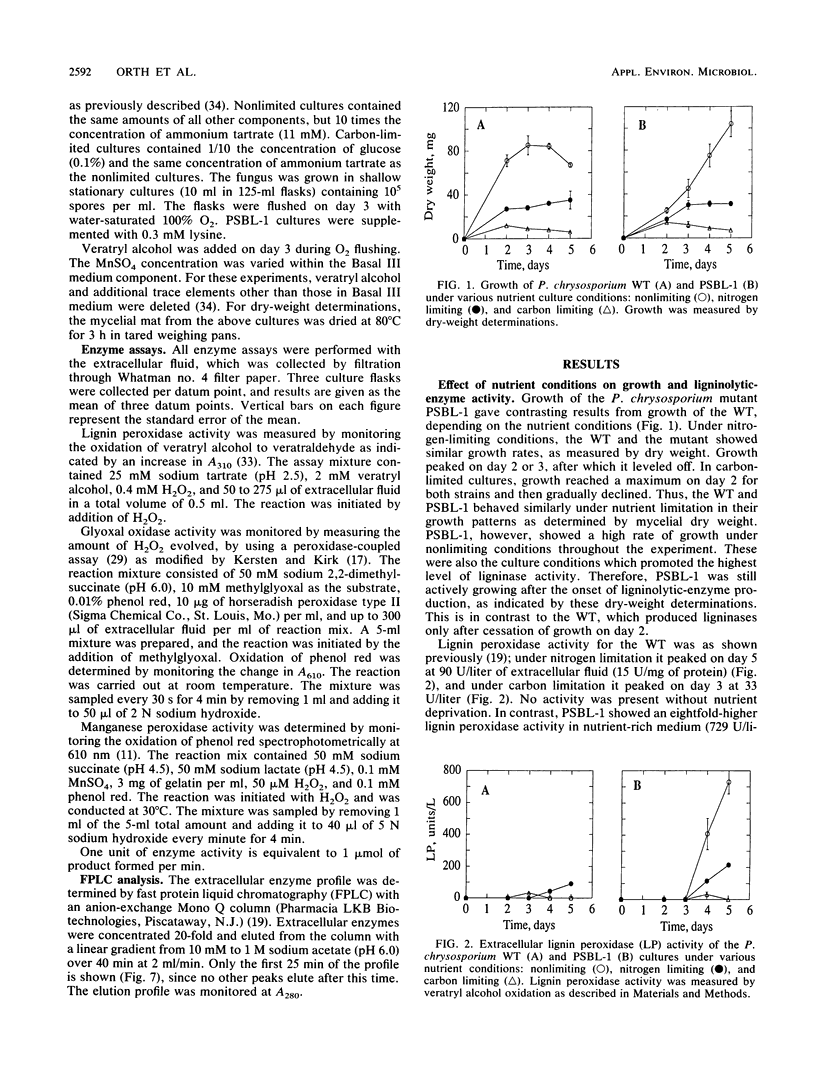
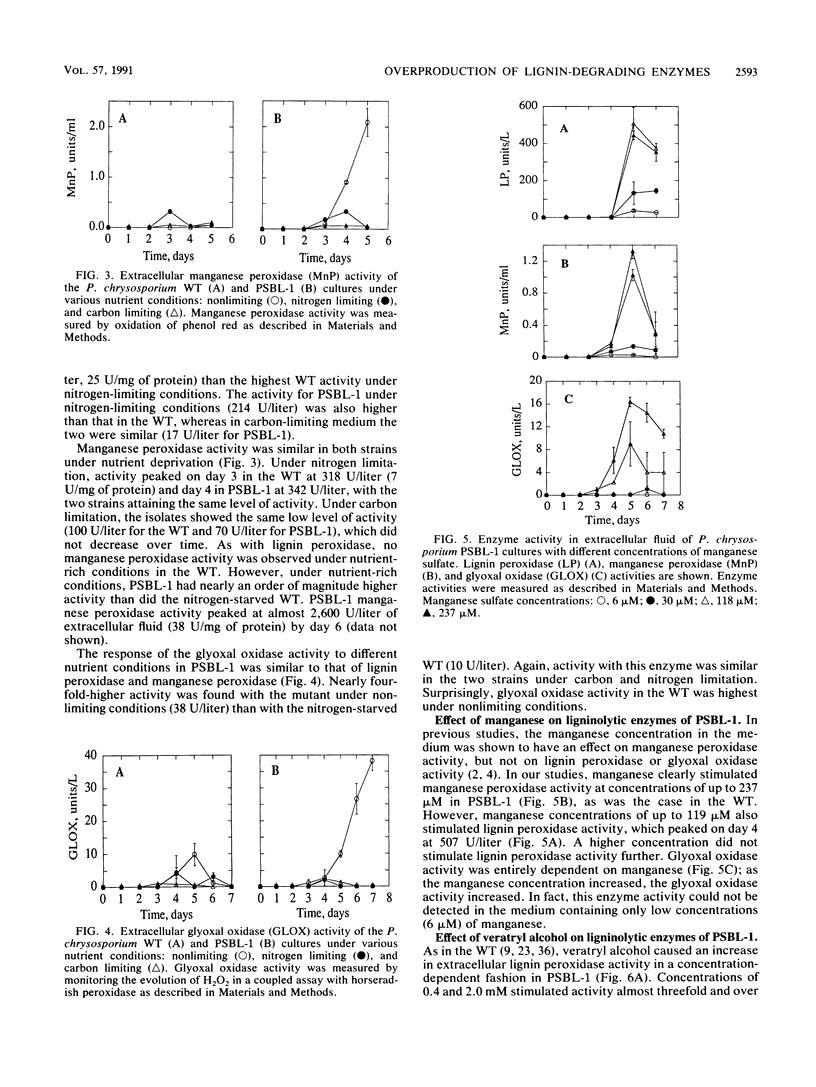
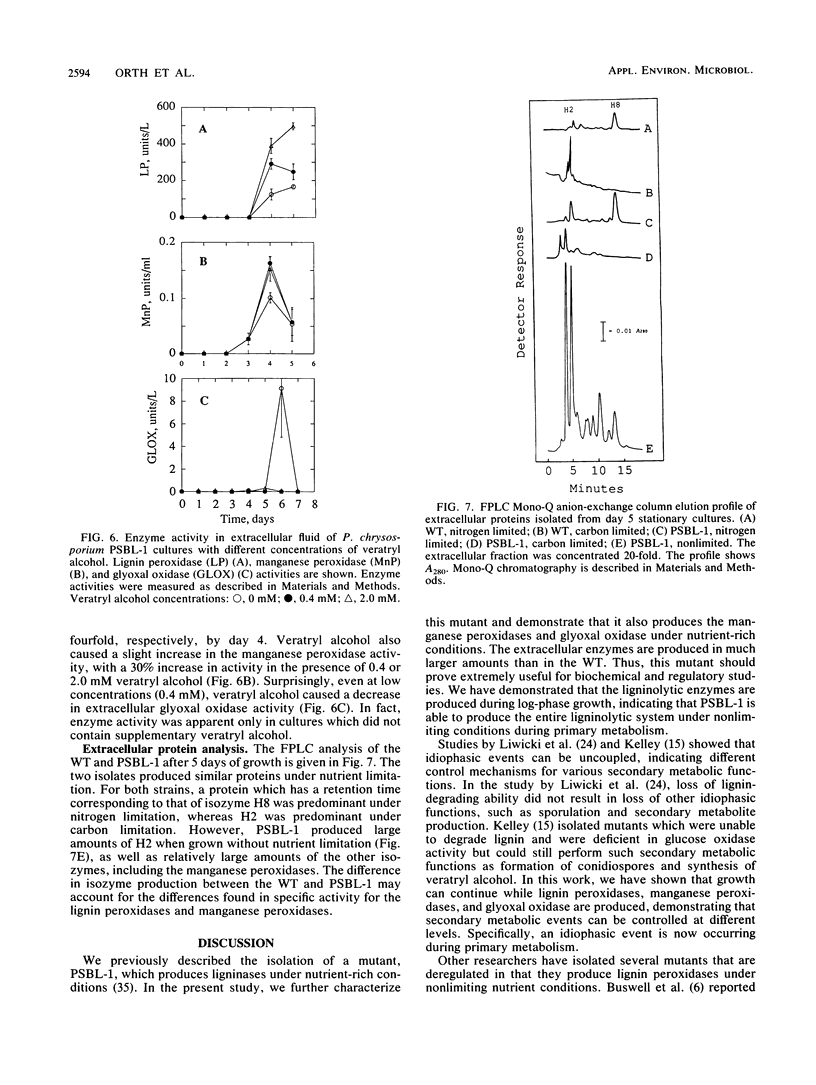
Phanerochaete chrysosporium is a white rot fungus which secretes a family of lignin-degrading enzymes under nutrient limitation. PSBL-1 is a mutant of this organism that generates the ligninolytic system under nonlimiting conditions during primary metabolism. Lignin peroxidase, manganese peroxidase, and glyoxal oxidase activities for PSBL-1 under nonlimiting conditions were 4- to 10-fold higher than those of the wild type (WT) under nitrogen-limiting conditions. PSBL-1 was still in the log phase of growth while secreting the enzymes, whereas the WT had ceased to grow by this time. As in the WT, manganese(II) increased manganese peroxidase activity in the mutant. However, manganese also caused an increase in lignin peroxidase and glyoxal oxidase activities in PSBL-1. Addition of veratryl alcohol to the culture medium stimulated lignin peroxidase activity, inhibited glyoxal oxidase activity, and had little effect on manganese peroxidase activity in PSBL-1, as in the WT. Fast protein liquid chromatography (FPLC) analysis shows production of larger amounts of isozyme H2 in PSBL-1 than in the WT. These properties make PSBL-1 very useful for isolation of large amounts of all ligninolytic enzymes for biochemical study, and they open the possibility of scale-up production for pratical use.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Reddy C. A. Nitrogen-deregulated mutants of Phanerochaete chrysosporium--a lignin-degrading basidiomycete. Arch Microbiol. 1990;153(6):521–527. doi: 10.1007/BF00245259. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Tien M., Wright D., Aust S. D. Oxidation of persistent environmental pollutants by a white rot fungus. Science. 1985 Jun 21;228(4706):1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liwicki R., Paterson A., MacDonald M. J., Broda P. Phenotypic classes of phenoloxidase-negative mutants of the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1985 May;162(2):641–644. doi: 10.1128/jb.162.2.641-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Regulation of nitrogen metabolism and gene expression in fungi. Microbiol Rev. 1981 Sep;45(3):437–461. doi: 10.1128/mr.45.3.437-461.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Tien M., Kersten P. J., Kirk T. K. Selection and improvement of lignin-degrading microorganisms: potential strategy based on lignin model-amino Acid adducts. Appl Environ Microbiol. 1987 Feb;53(2):242–245. doi: 10.1128/aem.53.2.242-245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


